Question: How equation 4 generate by substituting equation 1 and equation 2 in equation 3. Show me the details work please. 2, FAD (C-Cc) c (C
How equation 4 generate by substituting equation 1 and equation 2 in equation 3. Show me the details work please.
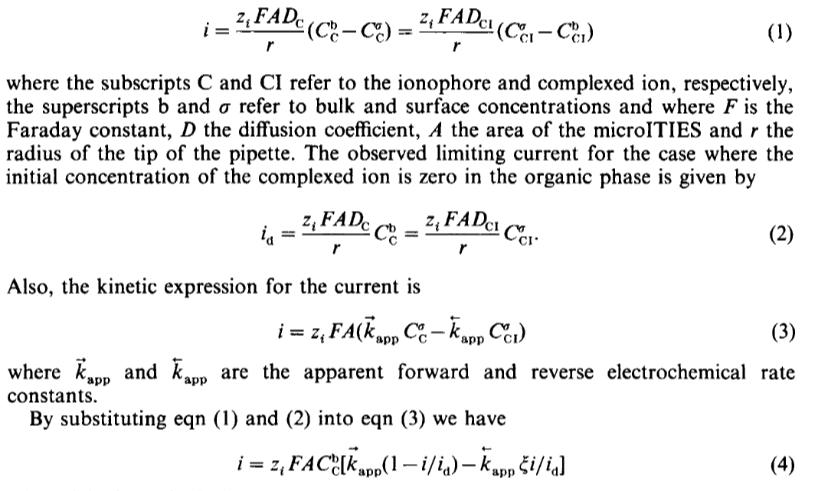
2, FAD (C-Cc) c (C - C ) = 2; FAD c (CC) - r (1) where the subscripts C and CI refer to the ionophore and complexed ion, respectively, the superscripts b and refer to bulk and surface concentrations and where F is the Faraday constant, D the diffusion coefficient, A the area of the microITIES and r the radius of the tip of the pipette. The observed limiting current for the case where the initial concentration of the complexed ion is zero in the organic phase is given by z, FADC ZFADCI ia C CCI r (2) Also, the kinetic expression for the current is i=z, FA(kapp C-kapp CC1) (3) where Kapp and kapp are the apparent forward and reverse electrochemical rate constants. By substituting eqn (1) and (2) into eqn (3) we have i=z, FAC[kapp(1-i/ia) - kapp i/ia] (4)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


