Question: how to do part c in excel 4. In a chemical reactor, reactant A is consumed to form products B and D. The reaction follows
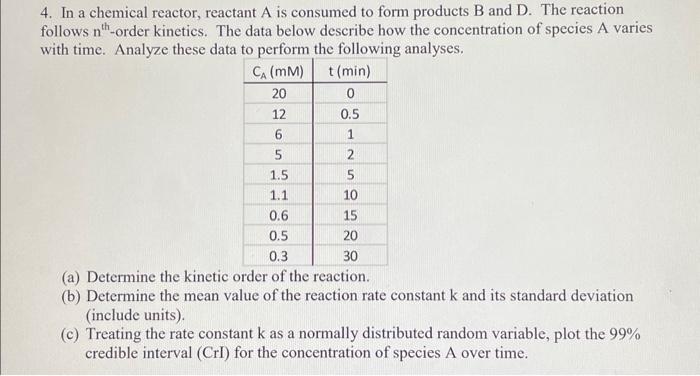
4. In a chemical reactor, reactant A is consumed to form products B and D. The reaction follows nth-order kinetics. The data below describe how the concentration of species A varies with time. Analyze these data to perform the following analyses. (a) Determine the kinetic order of the reaction. (b) Determine the mean value of the reaction rate constant k and its standard deviation (include units). (c) Treating the rate constant k as a normally distributed random variable, plot the 99% credible interval (CrI) for the concentration of species A over time
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


