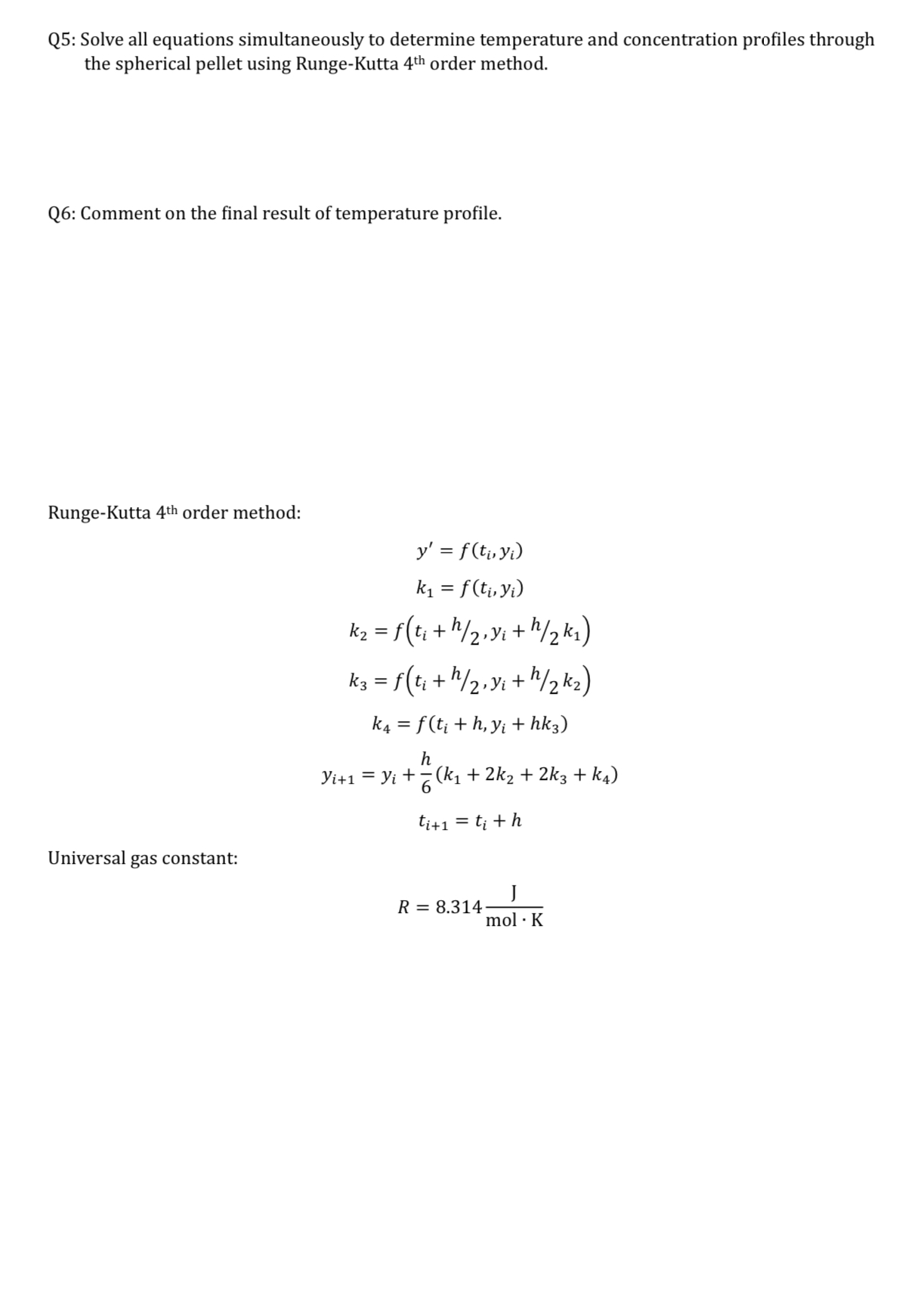
Question: How to solve Consider the case of a single first order reaction taking place in a non - isothermal spherical catalyst particle at steady state
How to solve
Consider the case of a single first order reaction taking place in a nonisothermal spherical catalyst particle at steady state without external mass or heat transfer resistance. We may assume constant values of heat of reaction, activation energy, and solid thermal conductivity and gas diffusion coefficient based on expected limited temperature variation. A requirement for catalytic surface integrity that the core temperature should not exceed
The chemical reaction is exothermic. Given the following conditions:
: Center maximum allowable Temperature
: rate constant at surface temperature
: reaction activation energy
: heat of reaction exothermic
: Diffusion coefficient of
: thermal conductivity of solid pellet
: radius of solid pellet
: Surface concentration of A Liter Liter :
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


