Question: https://www.compadre.org/Physlets/thermodynamics/ex20_3.cfmSolve please Worksheet for Exploration 20.3: Ideal Gas Law The relationship between the number of particles in a gas, the volume of the container holding
https://www.compadre.org/Physlets/thermodynamics/ex20_3.cfmSolve please
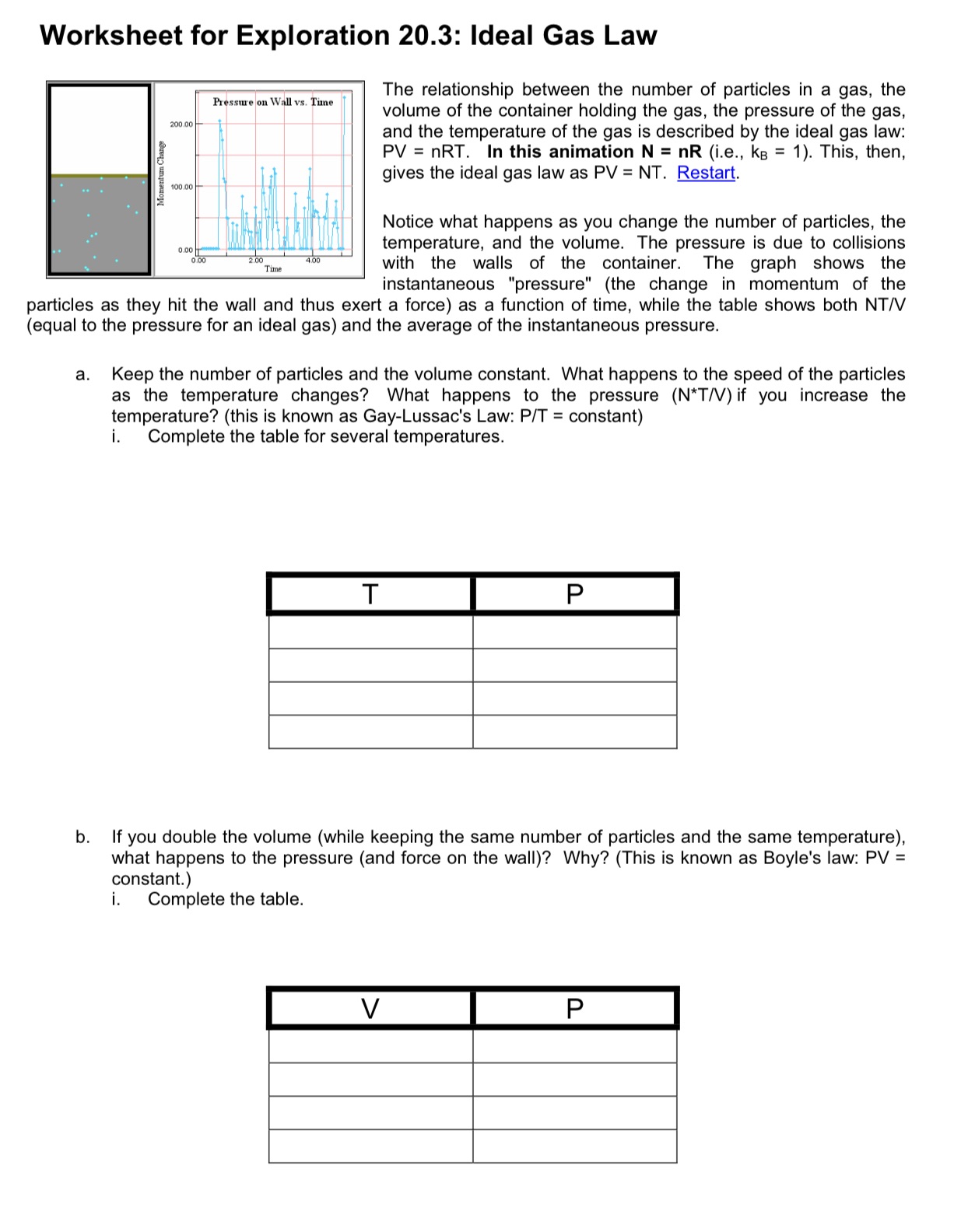
Worksheet for Exploration 20.3: Ideal Gas Law The relationship between the number of particles in a gas, the volume of the container holding the gas, the pressure of the gas, and the temperature of the gas is described by the ideal gas law: PV = nRT. In this animation N = nR (i.e., k3 = 1). This, then, gives the ideal gas law as PV = NT. Restart. Piesstu r an Wall vs Time Notice what happens as you change the number of particles, the temperature, and the volume. The pressure is due to collisions with the walls of the container. The graph shows the instantaneous "pressure" (the change in momentum of the particles as they hit the wall and thus exert a force) as a function of time, while the table shows both NTN (equal to the pressure for an ideal gas) and the average of the instantaneous pressure. a. Keep the number of particles and the volume constant. What happens to the speed of the particles as the temperature changes? What happens to the pressure (N*TN) if you increase the temperature? (this is known as Gay-Lussac's Law: PIT = constant) i. Complete the table for several temperatures. b. If you double the volume (while keeping the same number of particles and the same temperature), what happens to the pressure (and force on the wall)? Why? (This is known as Boyle's law: PV = constant.) i. Complete the table
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


