Question: Hydrogen Oxygen Cathode Anode Battery 2 H2O(l) + 2 H (9) + O2(9) The reaction in which H2O(l) is decomposed into H (m) and o(a)
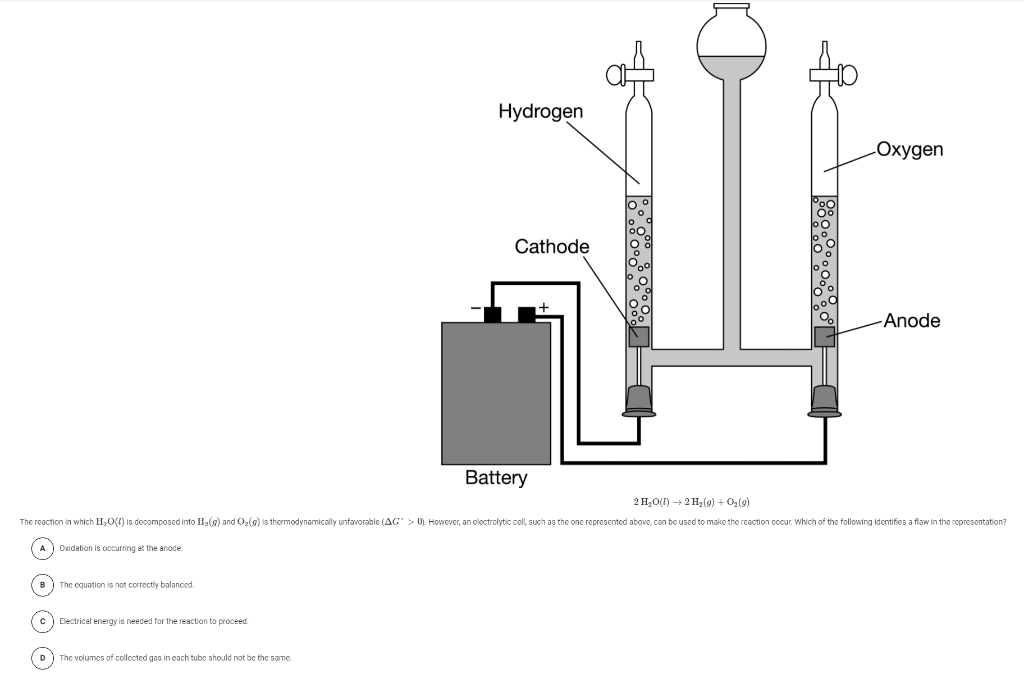
Hydrogen Oxygen Cathode Anode Battery 2 H2O(l) + 2 H (9) + O2(9) The reaction in which H2O(l) is decomposed into H (m) and o(a) is thermodynamically unfavorable (AG'>0). However, an electrolytic cell, such as the one represented above, can be used to make the reaction occur. Which of the following identifies a flaw in the representation? Oxidation is occurring at the anode. B The equation is not correctly balanced Electrical energy is needed for the reaction to proceed. D) The volumes of collected gas in cach tube should not be the same
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


