Question: I keep getting something wrong here and I am not sure what. Thanks for any help! In a study of the decomposition of nitrous oxide
I keep getting something wrong here and I am not sure what. Thanks for any help!
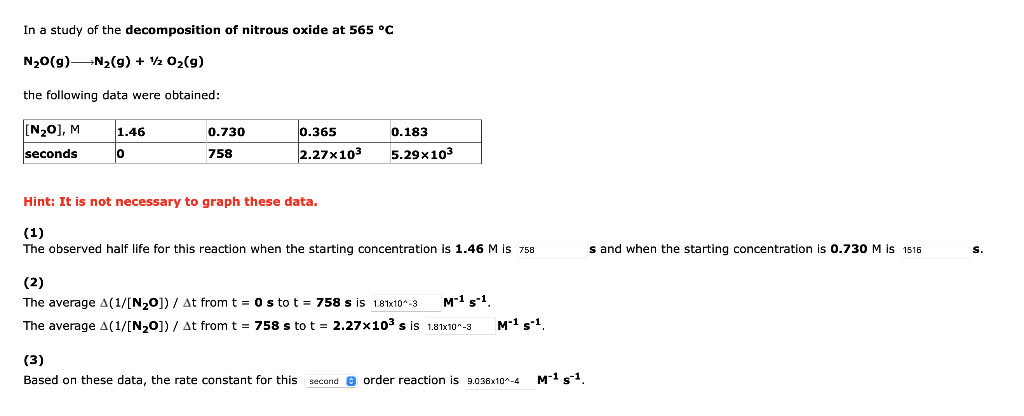
In a study of the decomposition of nitrous oxide at 565 C N20(9)N2(9) + 1/2O2(g) the following data were obtained: (N2O), M 1.46 0.730 0.365 0.183 seconds 0 758 2.27x103 5.29x103 Hint: It is not necessary to graph these data. (1) The observed half life for this reaction when the starting concentration is 1.46 M is 758 s and when the starting concentration is 0.730 M is 1516 s. (2) The average A(1/[N20]) / At from t = 0 s to t = 758 s is 1.81x10-3 Ms1 The average A(1/[N20]) / At from t = 758 s to t = 2.27x103 s is 1.81x10^-3 M-1 s-1 (3) Based on these data, the rate constant for this second order reaction is 9.038x10^-4 M-1 5-1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


