Question: I need help figuring out these problems. if you could please help that would be lovely and will you please write clearly. Thank you Problem
I need help figuring out these problems. if you could please help that would be lovely and will you please write clearly. Thank you
Problem #1
A series of standards were made as described in Table 1. A 0.2240 g sample of ore was digested in 25 mL of concentrated nitric acid for 10 minutes. The resulting solution (solution X1) was diluted with water to a total volume of 100.0 mL. A 20.00 mL aliquot of solution X1 was mixed with potassium ferrocyanide to produce a blue color from the complex formed with Fe3+ ion and this second solution (X2) was diluted to a total volume of 50.00 mL. The absorbance of solution X2 was 0.517. What is the percent iron in the ore, with error?
Table 1. Standards for the visible spectroscopic analysis of iron. [Fe3+]stock = 104.8 ppm. Total volume = 25.00 mL. reagent = 1 M K4Fe(CN)6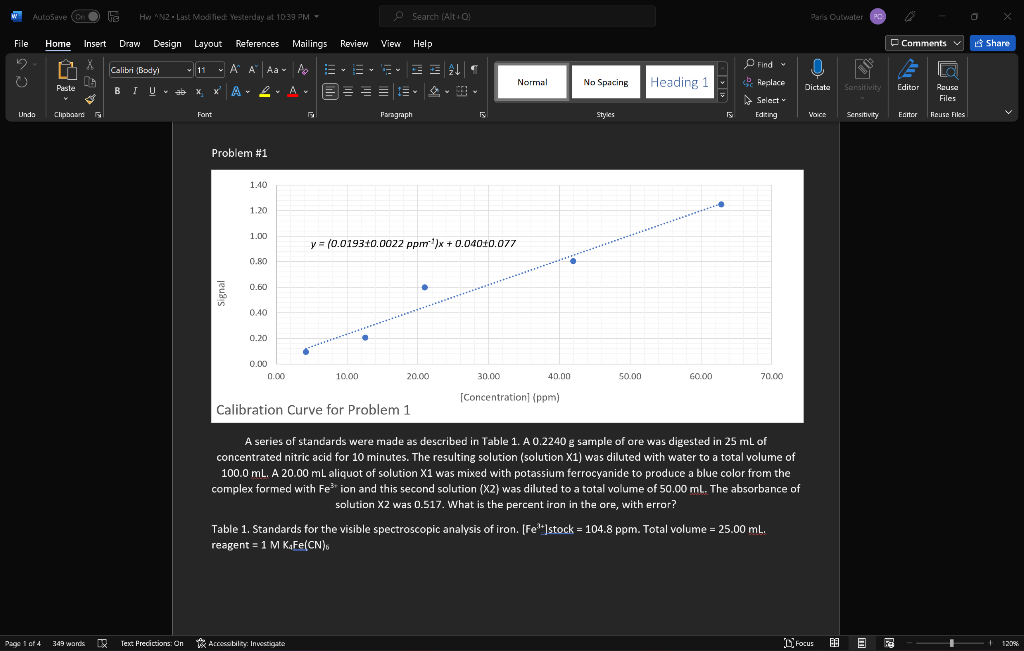
AutoSave (on 5 HwN2Last Modified: Yesterday at 10:39 PM - Search (Alto) Paris Outwater File Home Insert Draw Design Layout References Mailings Review View Help Comments Share Calibri Body) 11 A A Aa- ' ' BIU * XX A ab A ! A EEEEE Normal No Spacing Heading 1 U Dictate Paste Find Replace Select Editing Sensitivity Editor 1 Rouse Files Unda Clipboard Fant F Paragraph Styles VOICE Sensitivity Editor Reuse Files Problem #1 1.40 1.20 1.00 y = (0.019310.0022 ppm)x+ 0.04010.077 0.80 0.60 0.40 0.20 0.00 0.00 10.00 20.00 30.00 40.00 50.00 60.00 70.00 [Concentration (ppm) Calibration Curve for Problem 1 A series of standards were made as described in Table 1. A 0.2240 g sample of ore was digested in 25 mL of concentrated nitric acid for 10 minutes. The resulting solution (solution X1) was diluted with water to a total volume of 100.0 ml. A 20.00 mL aliquot of solution X1 was mixed with potassium ferrocyanide to produce a blue color from the complex formed with Festion and this second solution (X2) was diluted to a total volume of 50.00 ml. The absorbance of solution X2 was 0.517. What is the percent iron in the ore, with error? ? Table 1. Standards for the visible spectroscopic analysis of iron. [Fe']stock = 104.8 ppm. Total volume = 25.00 ml. reagent = 1 M KAFe(CN). Page 1 of 4 349 wands lex Predictions: On Accessibility Investigate Focus BE E + 1208
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


