Question: i need help for the three questions A buffer solution is 0.328M in HF and 0.393M in KF. If Ka for HF is 7.2104, what
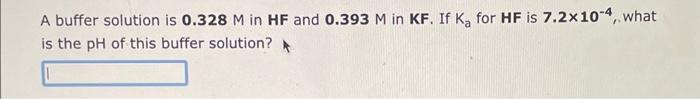


A buffer solution is 0.328M in HF and 0.393M in KF. If Ka for HF is 7.2104, what is the pH of this buffer solution? A buffer solution is 0.329M in HNO2 and 0.320M in KNO2. If Ka for HNO2 is 4.5104, what is the pH of this buffer solution? A buffer solution is 0.489M in CH3COOH and 0.265M in CH3COOK. If Ka for CH3COOH is 1.8105, what is the pH of this buffer solution
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


