Question: i need help for the three questions A buffer solution contains 0.384MC6H5NH3Cl and 0.253MC6H5NH2 (aniline). Determine the pH change when 0.073molNaOH is added to 1.00L
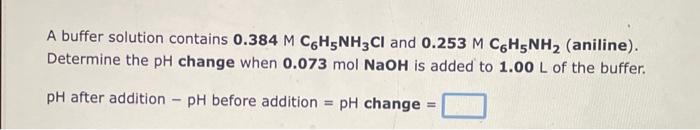


A buffer solution contains 0.384MC6H5NH3Cl and 0.253MC6H5NH2 (aniline). Determine the pH change when 0.073molNaOH is added to 1.00L of the buffer. pH after addition pH before addition =pH change = Determine the pH change when 0.067molHNO3 is added to 1.00L of a buffer solution that is 0.329M in HNO2 and 0.320M in NO2. pH after addition pH before addition =pH change = Determine the pH change when 0.130molKOH is added to 1.00L of a buffer solution that is 0.489M in CH3COOH and 0.265M in CH3COO. pH after addition pH before addition =pH change =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


