Question: I NEED HELP ON BOTH PLEASE Suppose experiments show that if the chemical reaction N2O52NO2+21O2 takes place at a certain temperature, the rate of reaction
I NEED HELP ON BOTH PLEASE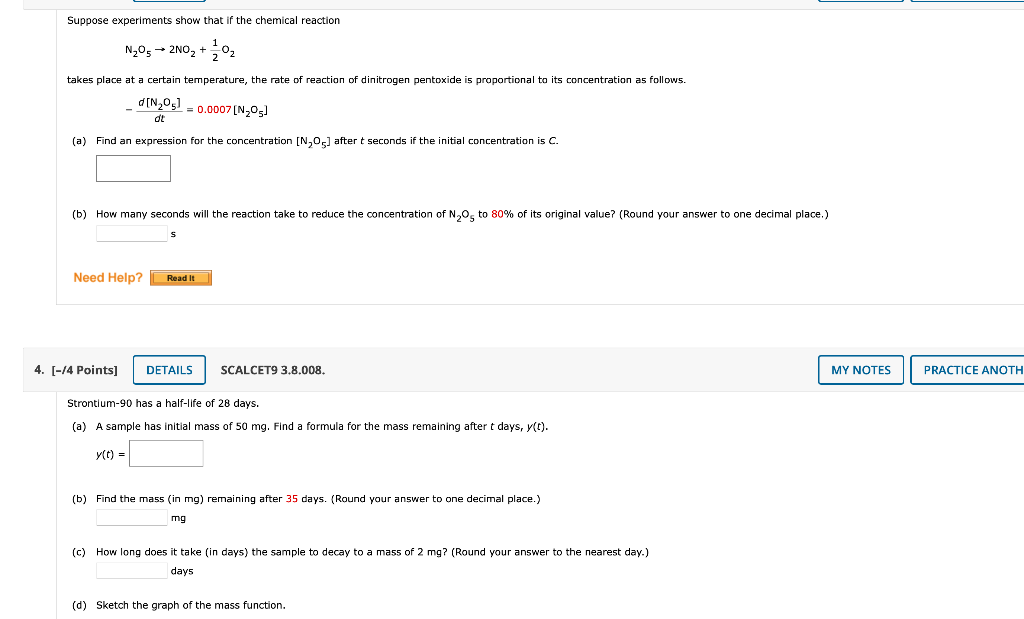
Suppose experiments show that if the chemical reaction N2O52NO2+21O2 takes place at a certain temperature, the rate of reaction of dinitrogen pentoxide is proportional to its concentration as follows. dtd[N2O5]=0.0007[N2O5] (a) Find an expression for the concentration [N2O5] after t seconds if the initial concentration is C. (b) How many seconds will the reaction take to reduce the concentration of N2O5 to 80% of its original value? (Round your answer to one decimal place.) 5 /4 Points] SCALCET9 3.8.008. Strontium-90 has a half-life of 28 days. (a) A sample has initial mass of 50mg. Find a formula for the mass remaining after t days, y(t). y(t)= (b) Find the ma55 (in mg) remaining after 35 days. (Round your answer to one decimal place.) mg (c) How long does it take (in days) the sample to decay to a mas5 of 2mg ? (Round your answer to the nearest day.) days (d) Sketch the graph of the mass function
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


