Question: i Need help on this problem a step by step explanation for 1 , 2 , and 3 would be helpful. I will leave a
i Need help on this problem a step by step explanation for and would be helpful. I will leave a like
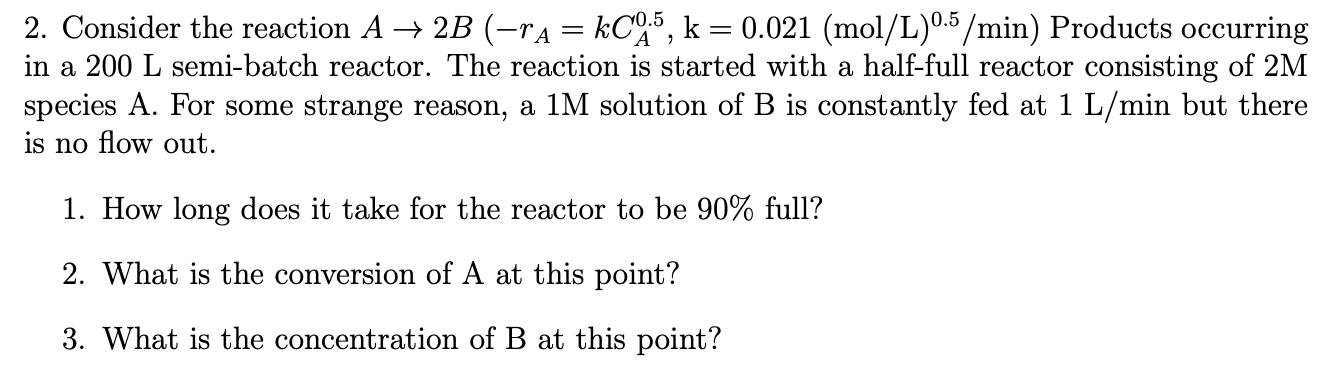
Consider the reaction Products occurring
in a semibatch reactor. The reaction is started with a halffull reactor consisting of
species A For some strange reason, a solution of is constantly fed at but there
is no flow out.
How long does it take for the reactor to be full?
What is the conversion of at this point?
What is the concentration of at this point?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


