Question: i need help with questions 1-3 please 1. Write the ionic equation for the precipitation reaction. 2. Explain why the AgCl precipitate dissolved when NH3
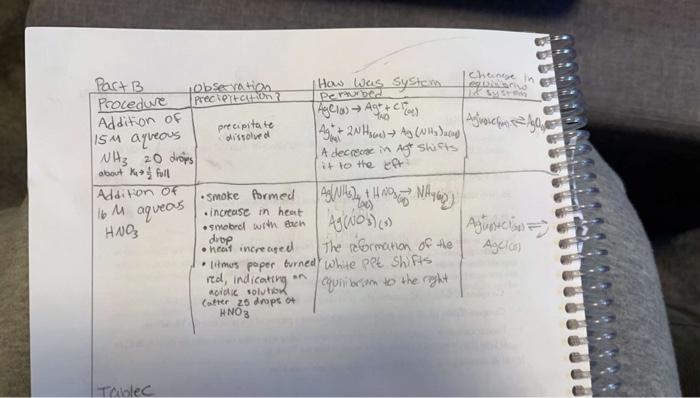
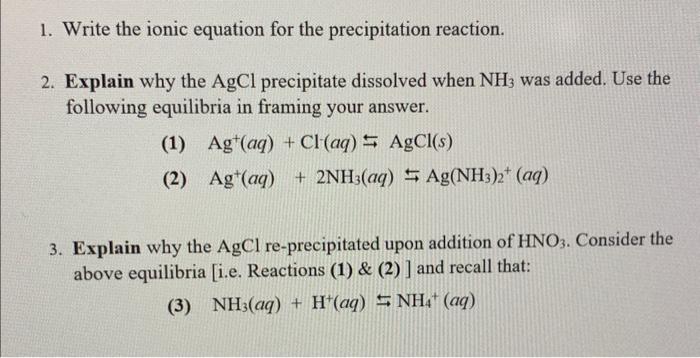
1. Write the ionic equation for the precipitation reaction. 2. Explain why the AgCl precipitate dissolved when NH3 was added. Use the following equilibria in framing your answer. (1) Ag+(aq)+Cl(aq)AgCl(s) (2) Ag+(aq)+2NH3(aq)Ag(NH3)2+(aq) 3. Explain why the AgCl re-precipitated upon addition of HNO3. Consider the above equilibria [i.e. Reactions (1) \& (2)] and recall that: (3) NH3(aq)+H+(aq)NH4+(aq)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


