Question: A polytropic process is a thermonic process that obeys the relation: pV constant The exponent a is known as the polytropic index, and it
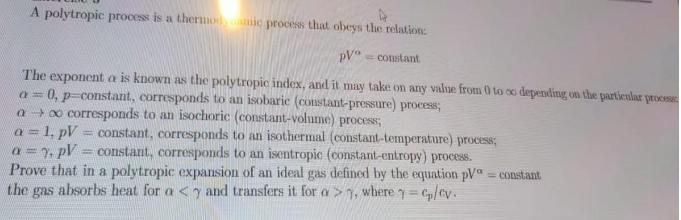
A polytropic process is a thermonic process that obeys the relation: pV constant The exponent a is known as the polytropic index, and it may take on any value from 0 to co depending on the particular process a= 0, p-constant, corresponds to an isobaric (constant-pressure) process; a o corresponds to an isochoric (constant-volume) process; a = 1, pV = constant, corresponds to an isothermal (constant-temperature) process; a = y, pV = constant, corresponds to an isentropic (constant-entropy) process. Prove that in a polytropic expansion of an ideal gas defined by the equation pV = constant the gas absorbs heat for a , where = cp/cv.
Step by Step Solution
3.45 Rating (161 Votes )
There are 3 Steps involved in it
To tackle this problem we need to understand the concept of a polytropic process for an ideal gas an... View full answer

Get step-by-step solutions from verified subject matter experts


