Question: I need the answer under 25 min please. just the final answer ( no explaination ) For series reaction in batch reactor, the following curve
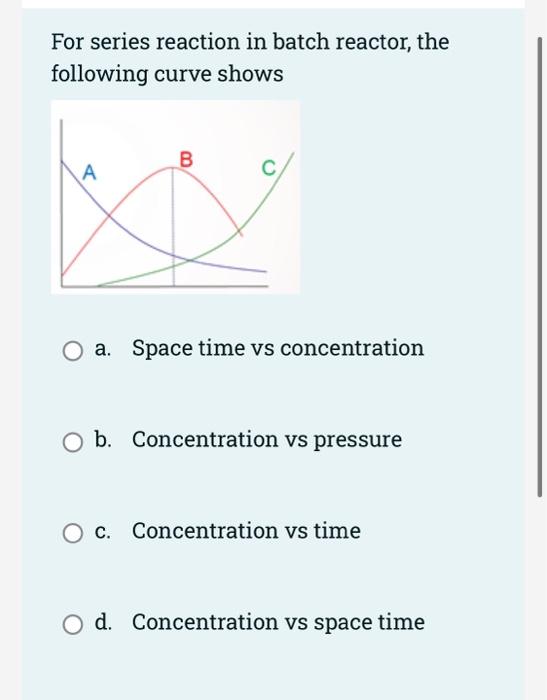
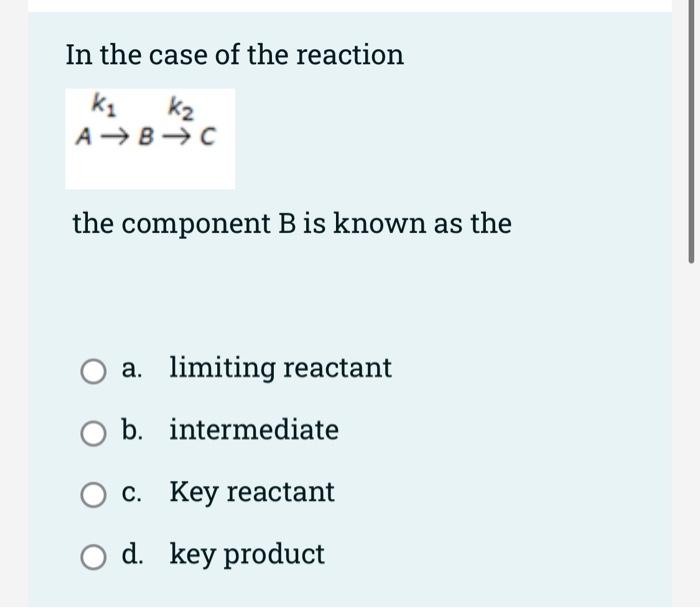
For series reaction in batch reactor, the following curve shows B. A A O a. Space time vs concentration O b. Concentration vs pressure O c. C. Concentration vs time O d. Concentration vs space time In the case of the reaction ki k, A+B=0 ABC the component B is known as the a. limiting reactant O b. intermediate c. Key reactant d. key product
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


