Question: I WANT THE MATLAB CODE FOR THE QUESTION BELOW PLS. Assume you have an adiabatic CSTR operating at steady state. Within the CSTR, reactant A
I WANT THE MATLAB CODE FOR THE QUESTION BELOW PLS.
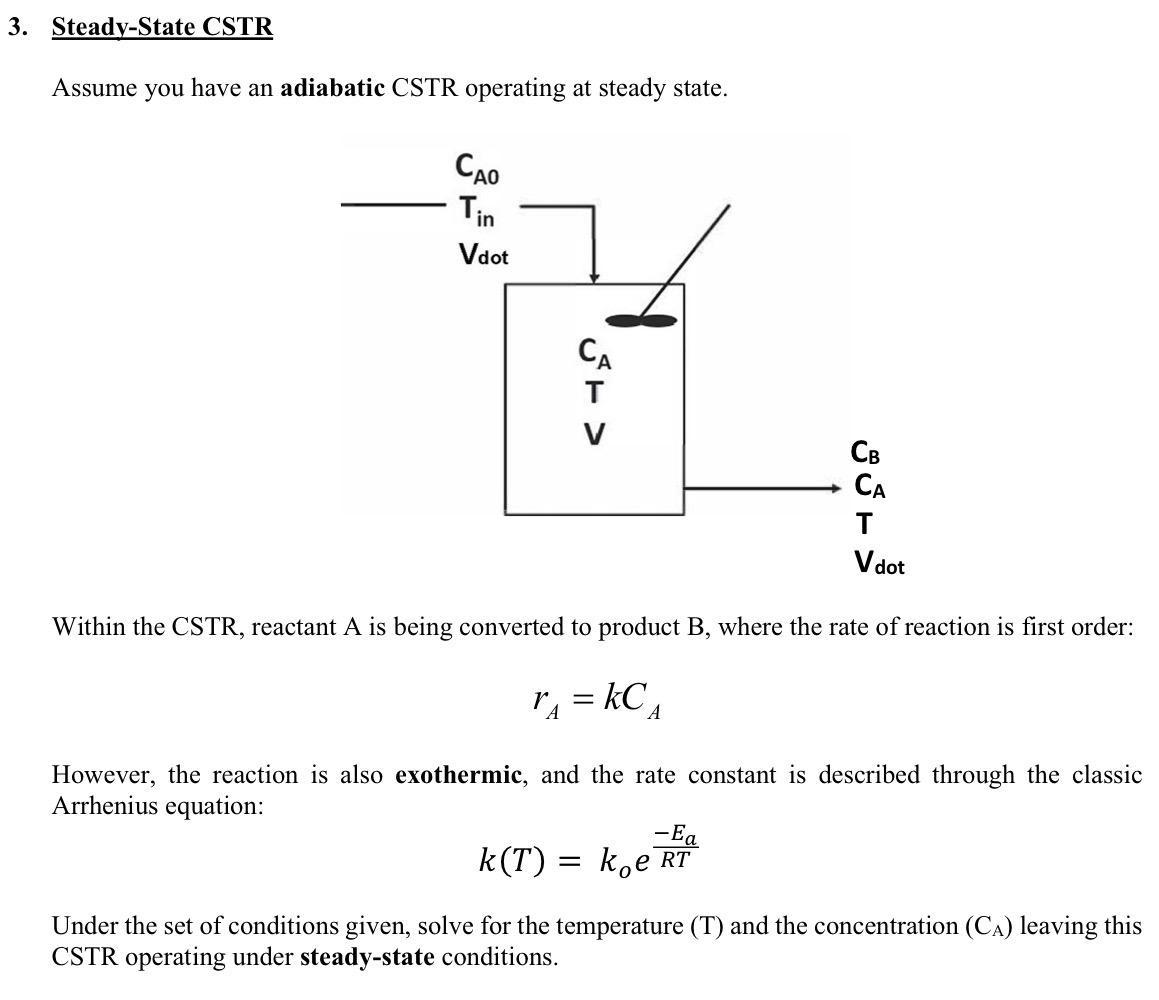

Assume you have an adiabatic CSTR operating at steady state. Within the CSTR, reactant A is being converted to product B, where the rate of reaction is first order: rA=kCA However, the reaction is also exothermic, and the rate constant is described through the classic Arrhenius equation: k(T)=koeRTEa Under the set of conditions given, solve for the temperature (T) and the concentration (CA) leaving this CSTR operating under steady-state conditions. a. Write out the mass balance for A Assumptions: i. Assume input stream is pure CA0. ii. Assume constant density. b. Write out the energy balance. Assumptions: i. Assume no shaft work. ii. Negligible changes in kinetic and potential energy of system. iii. Adiabatic reactor (Q=0). iv. Cp is the same for both reactants and products. v. Assume stoichiometry for the reaction is 1:1. Hints: Note that we have a temperature change and a chemical reaction when you are writing out and simplifying your energy balance. c. Use MATLAB's f solve function to solve for the reactor temperature and concentration. Use an initial guess of 5mol/L for CA and an initial guess of 700K for temperature. d. Display the f solve results for CA and Tout using both disp and fprintf. Don't forget to include units
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


