Question: If the freezing point depression for a solution is 2.5C and Kf=4.5C/m, what is the molality of the solution? Select one: 6.0m 11min 0.56m 20m
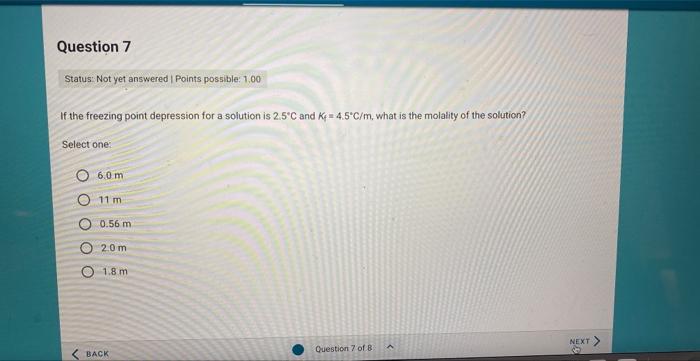
If the freezing point depression for a solution is 2.5C and Kf=4.5C/m, what is the molality of the solution? Select one: 6.0m 11min 0.56m 20m 1.8m
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


