Question: If the reaction sequence given below is carried out with 15 moles of acetylene, the amount of the product D formed (in g) is
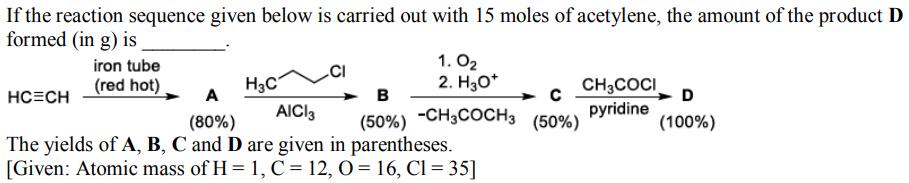
If the reaction sequence given below is carried out with 15 moles of acetylene, the amount of the product D formed (in g) is iron tube (red hot) HC=CH H3C 1.0 2. H3O+ A (80%) AICI3 The yields of A, B, C and D are given in parentheses. [Given: Atomic mass of H= 1, C = 12, O = 16, C1 = 35] CH3COCI pyridine B C (50%) -CH3COCH3 (50%) D (100%)
Step by Step Solution
There are 3 Steps involved in it
The detailed answer for t... View full answer

Get step-by-step solutions from verified subject matter experts


