Question: im confused on how to so these two problems ( they correspond with each other) 1 pts Question 3 In the reaction (needs to be
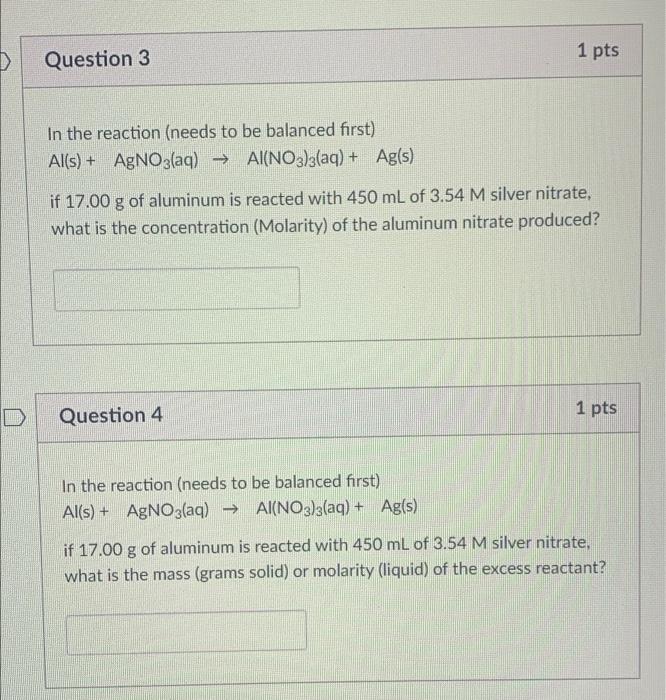
1 pts Question 3 In the reaction (needs to be balanced first) Al(s) + AgNO3(aq) + Al(NO3)3(aq) + Ag(s) ( if 17.00 g of aluminum is reacted with 450 mL of 3.54 M silver nitrate, what is the concentration (Molarity) of the aluminum nitrate produced? Question 4 1 pts In the reaction (needs to be balanced first) Al(s) + AgNO3(aq) + Al(NO3)3(aq) + Ag(s) s if 17.00 g of aluminum is reacted with 450 mL of 3.54 M silver nitrate, what is the mass (grams solid) or molarity (liquid) of the excess reactant
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


