Question: In a batch or batch reactor the reaction is carried out: A B, in the liquid phase. The data obtained are shown in the table.
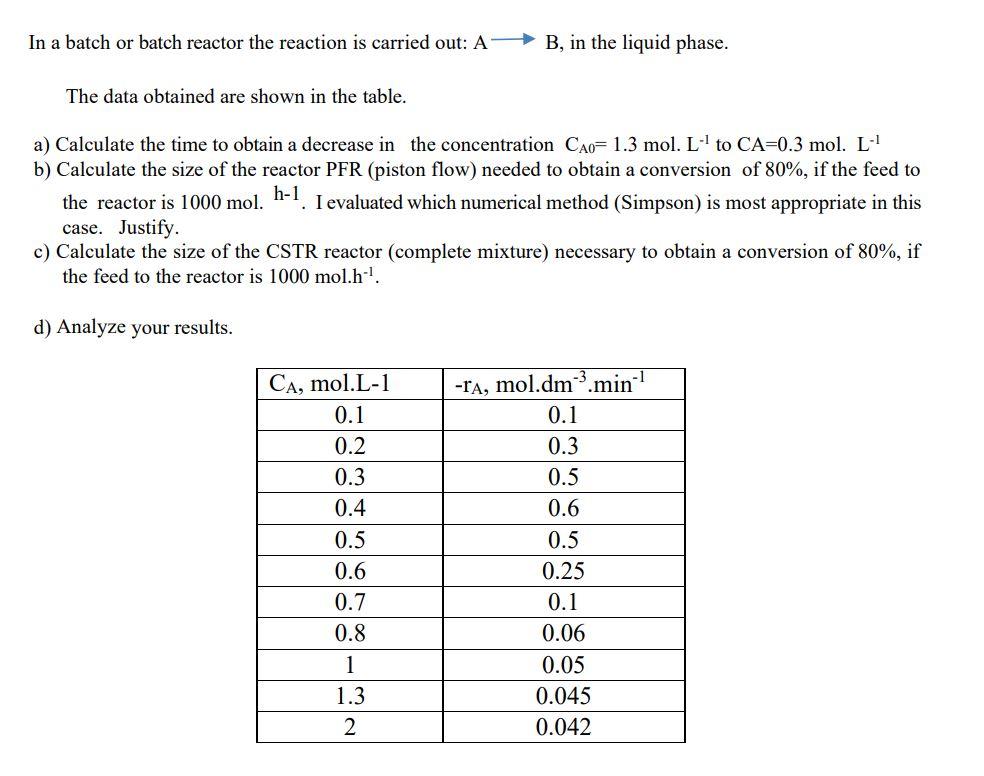
In a batch or batch reactor the reaction is carried out: A B, in the liquid phase. The data obtained are shown in the table. h-1 a) Calculate the time to obtain a decrease in the concentration CAo= 1.3 mol. L' to CA=0.3 mol. L- b) Calculate the size of the reactor PFR (piston flow) needed to obtain a conversion of 80%, if the feed to the reactor is 1000 mol. I evaluated which numerical method (Simpson) is most appropriate in this case. Justify. c) Calculate the size of the CSTR reactor (complete mixture) necessary to obtain a conversion of 80%, if the feed to the reactor is 1000 mol.h1. d) Analyze your results. -A, mol.dm.minh1 0.1 0.3 0.5 0.6 CA, mol.L-1 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 1 1.3 2 0.5 0.25 0.1 0.06 0.05 0.045 0.042
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


