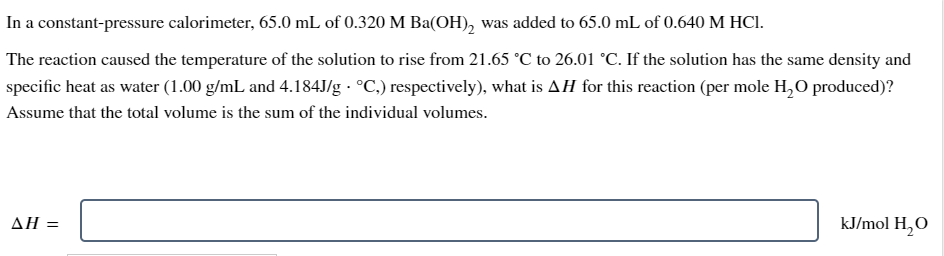
Question: In a constant - pressure calorimeter, 6 5 . 0 m L of 0 . 3 2 0 MBa ( O H ) 2 was
In a constantpressure calorimeter, of MBa was added to of
The reaction caused the temperature of the solution to rise from to If the solution has the same density and
specific heat as water and respectively what is for this reaction per mole produced
Assume that the total volume is the sum of the individual volumes.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


