Question: In a dilute solution, A decomposes to form B and C by the reaction: A B+C The rate law which describes the rate of this
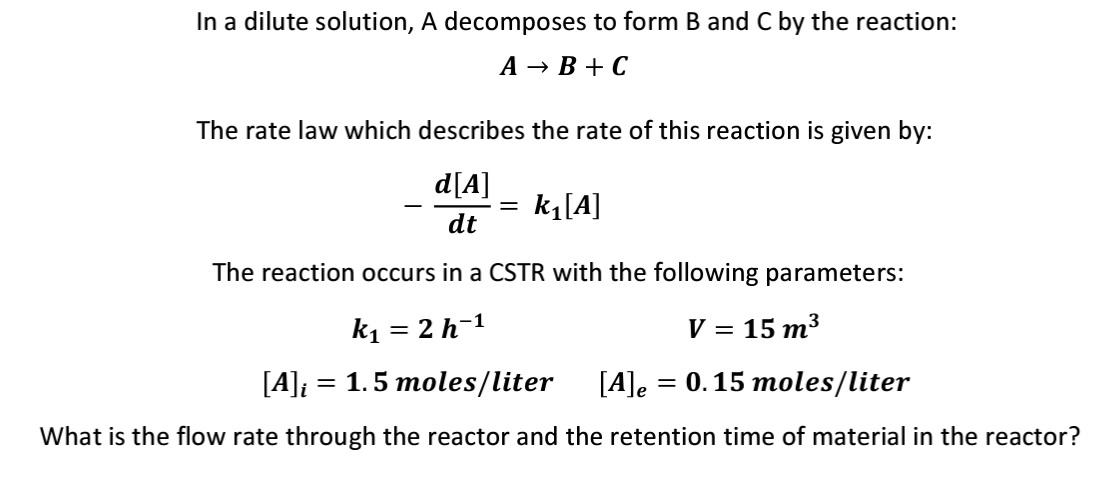
In a dilute solution, A decomposes to form B and C by the reaction: A B+C The rate law which describes the rate of this reaction is given by: d[A] dt = k[A] The reaction occurs in a CSTR with the following parameters: k1 = 2 h-1 V = 15 m3 = = [A]i = 1.5 moles/liter [A]e = 0.15 moles/liter What is the flow rate through the reactor and the retention time of material in the reactor
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


