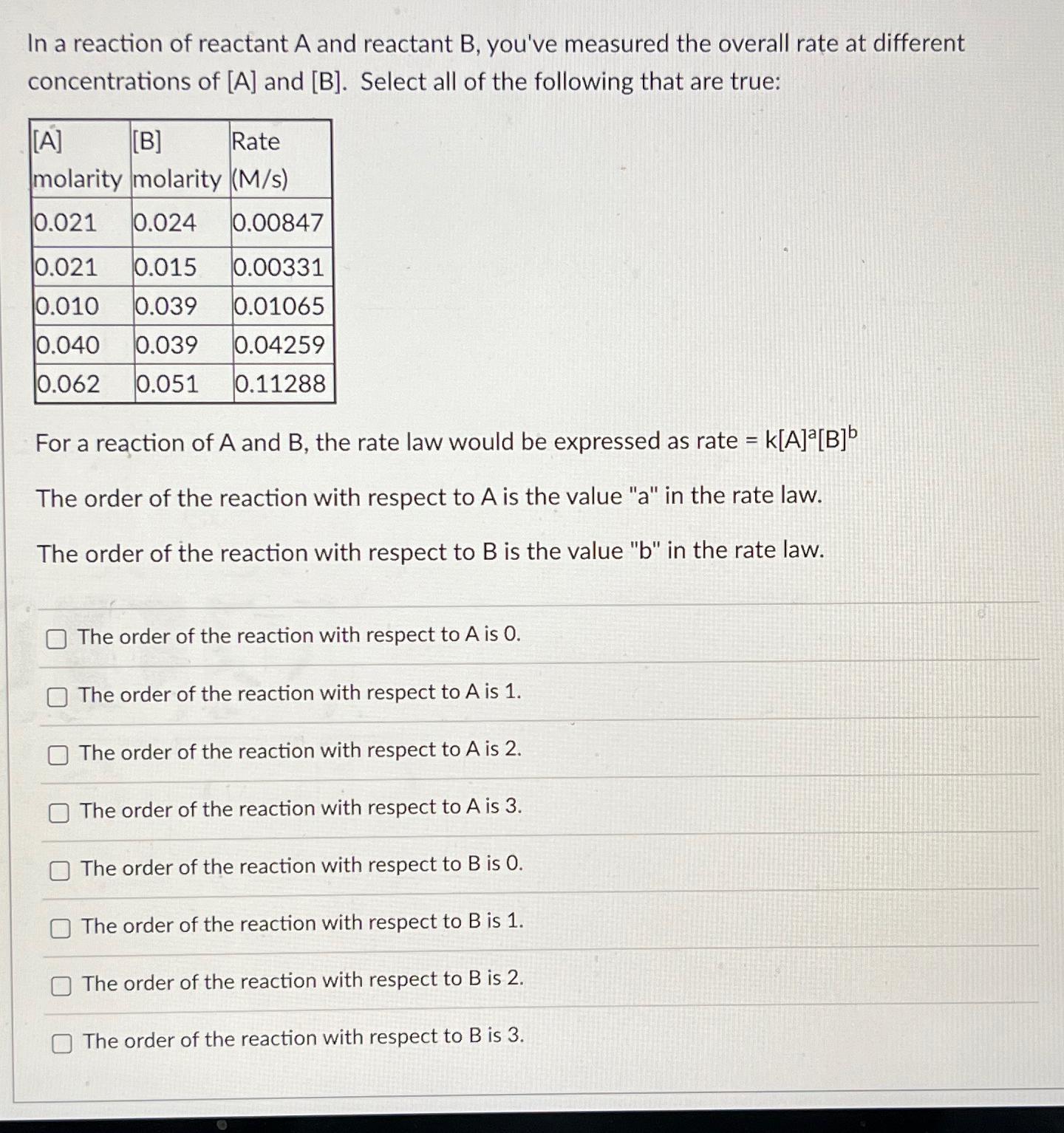
Question: In a reaction of reactant A and reactant B , you've measured the overall rate at different concentrations of A and B . Select all
In a reaction of reactant A and reactant you've measured the overall rate at different concentrations of A and Select all of the following that are true:
tabletableRatemolarity
For a reaction of A and the rate law would be expressed as rate
The order of the reaction with respect to is the value a in the rate law.
The order of the reaction with respect to is the value in the rate law.
The order of the reaction with respect to is
The order of the reaction with respect to is
The order of the reaction with respect to is
The order of the reaction with respect to is
The order of the reaction with respect to is
The order of the reaction with respect to is
The order of the reaction with respect to is
The order of the reaction with respect to is
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


