Question: In each compound below, two protons are color-coded (red and blue). Determine which of the two protons is more acidic, and explain your choice: The
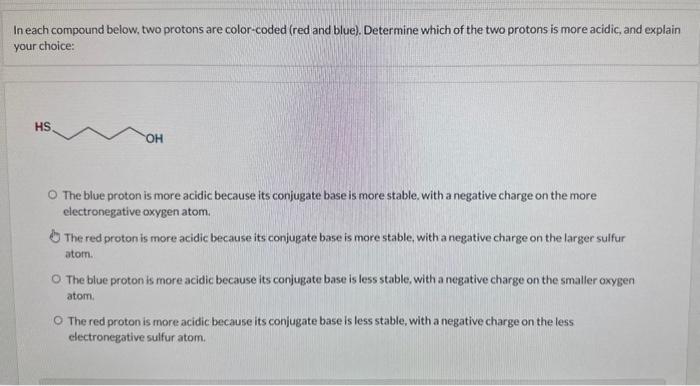
In each compound below, two protons are color-coded (red and blue). Determine which of the two protons is more acidic, and explain your choice: The blue proton is more acidic because its conjugate base is more stable. with a negative charge on the more electronegative oxygen atom. The red proton is more acidic because its conjugate base is more stable, with a negative charge on the larger sulfur atom. The blue proton is more acidic because its conjugate base is less stable, with a negative charge on the smaller oxygen atom. The red proton is more acidic because its conjugate base is less stable, with a negative charge on the less electronegative sulfur atom
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


