Question: Instructions: 1. Solve these problems using Microsoft Excel. 2. The final excel files must be uploaded to the blackboard (assignment section) before the deadline. 3.
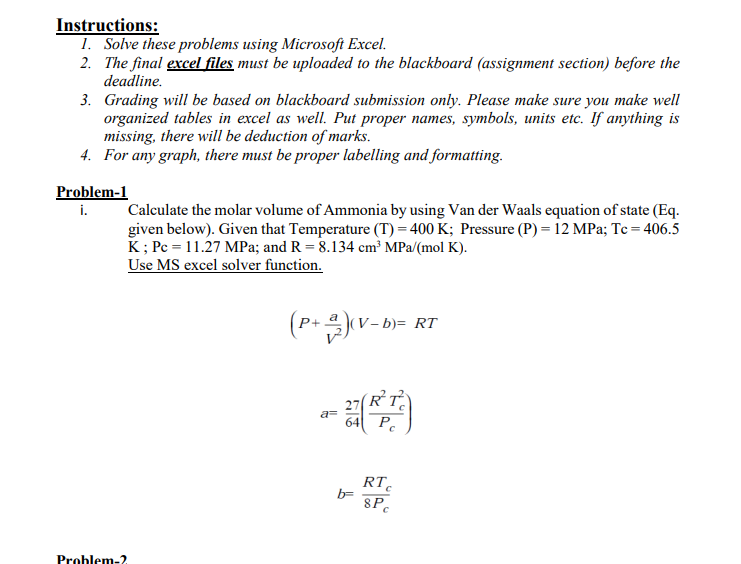
Instructions: 1. Solve these problems using Microsoft Excel. 2. The final excel files must be uploaded to the blackboard (assignment section) before the deadline. 3. Grading will be based on blackboard submission only. Please make sure you make well organized tables in excel as well. Put proper names, symbols, units etc. If anything is missing, there will be deduction of marks. 4. For any graph, there must be proper labelling and formatting. Problem-1 i. Calculate the molar volume of Ammonia by using Van der Waals equation of state (Eq. given below). Given that Temperature (T) = 400 K; Pressure (P)=12 MPa; Tc = 406.5 K; Pc = 11.27 MPa; and R = 8.134 cm MPa (mol K). Use MS excel solver function. (P+ 2)(V-b= (V-b)= RT RT a= 641 PC c b= RT 8PC Problem-2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


