Question: Instructions: Solve these problems using Microsoft Excel. Each Problem in a separate sheet within the same Excel workbook. The final Excel files must be uploaded
Instructions:
Solve these problems using Microsoft Excel. Each Problem in a separate sheet within the same Excel workbook.
The final Excel files must be uploaded to the blackboard assignment section before the deadline.
Submit the hardcopy if any to the TA at the end of the LAB session.
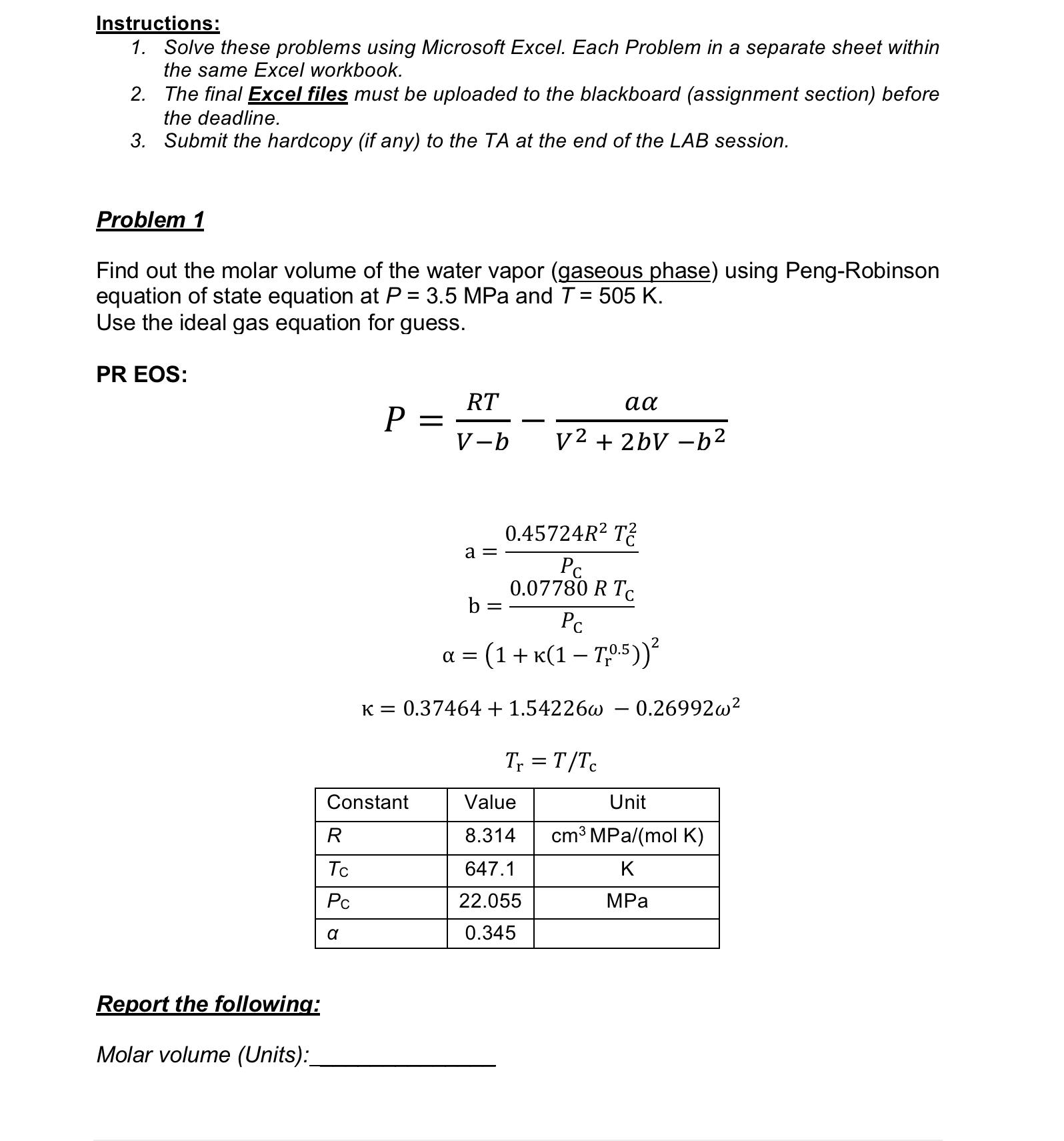
Problem
Find out the molar volume of the water vapor gaseous phase using PengRobinson equation of state equation at MPa and
Use the ideal gas equation for guess.
PR EOS:
tableConstantValue,Unit
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


