Question: A one litre solution contains 0.001 moles of CO ions and 0.01 moles of HCO, lons. What is the PH of the solution? [Ka,
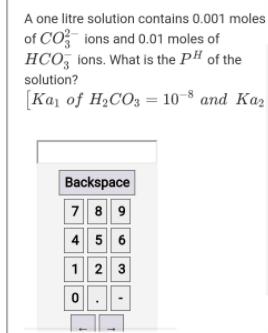
A one litre solution contains 0.001 moles of CO ions and 0.01 moles of HCO, lons. What is the PH of the solution? [Ka, of H2CO3 = 10-8 and Kaz Backspace 789 4 5 6 1 2 3
Step by Step Solution
3.49 Rating (159 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


