Question: Introduction to Chemical Engineering Thermodynamics eighth edition written, = d = ($(1) (10.67) ling correlations for $ and ot rather than for their logarithms. nd
Introduction to Chemical Engineering Thermodynamics eighth edition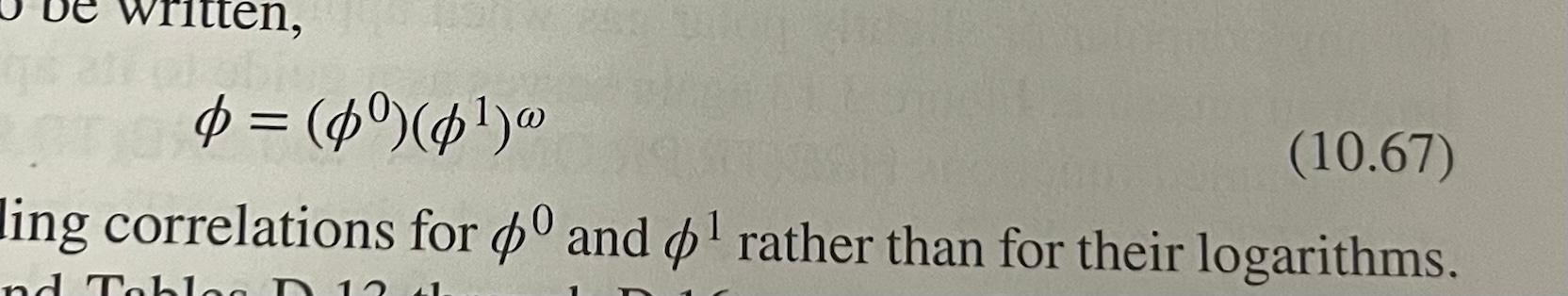
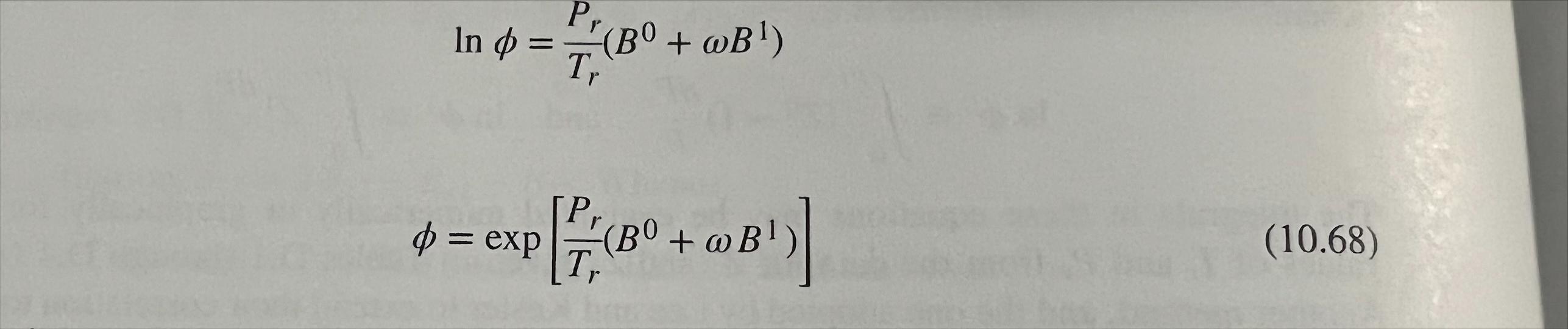
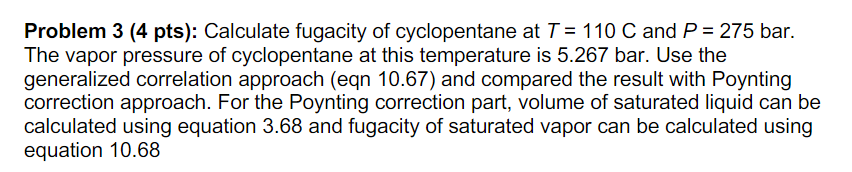
written, = d = ($(1) (10.67) ling correlations for $ and ot rather than for their logarithms. nd Tohino D 1711 P, In 0 = 0 "(B + @B) T, . : = exp (B + B!) P0:00+ . B!) (10.68) Problem 3 (4 pts): Calculate fugacity of cyclopentane at T = 110 C and P = 275 bar. The vapor pressure of cyclopentane at this temperature is 5.267 bar. Use the generalized correlation approach (eqn 10.67) and compared the result with Poynting correction approach. For the Poynting correction part, volume of saturated liquid can be calculated using equation 3.68 and fugacity of saturated vapor can be calculated using equation 10.68
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


