Question: Ionic conductivity of solutions. With the data from table 1 determine: a) The molar conductivity at infinite dilution for KCl. b)The degree of ionization of
Ionic conductivity of solutions. With the data from table 1 determine: a) The molar conductivity at infinite dilution for KCl. b)The degree of ionization of acetic acid at different concentrations and the ionization constant.
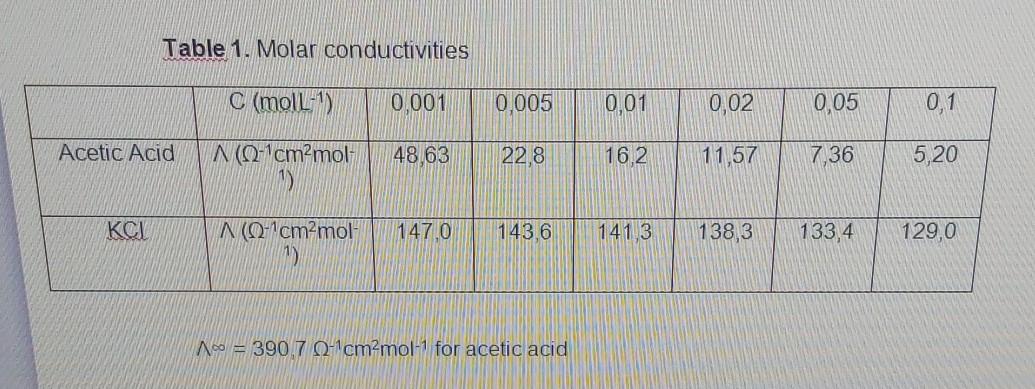
Table 1. Molar conductivities C (moll-) 0.001 0.005 0,01 0,02 0,05 0,1 Acetic Acid N (0-1cm2mol 48,63 22,8 16.2 11,57 7,36 5,20 KCI N (0-cm-mol 147,0 143.6 141.3 138,3 133,4 129,0 A = 390,7 0-cmmol-1 for acetic acid
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


