Question: It it sometimes necessary to convert the amount (in grams or milliliters) of a compound to moles. If a procedure required that you add 21.2

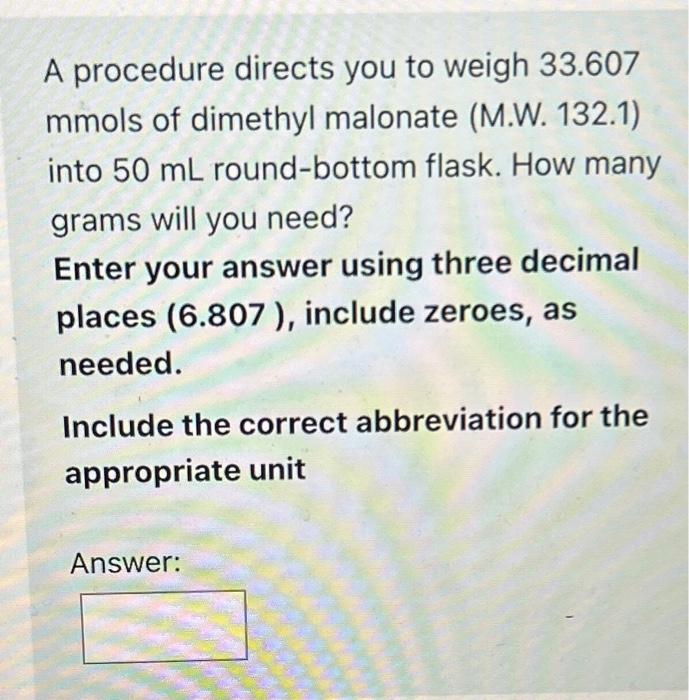
It it sometimes necessary to convert the amount (in grams or milliliters) of a compound to moles. If a procedure required that you add 21.2 grams of p toluenesulfonic acid (M.W. 172.2) to a reaction mixture, how many moles of this compound would you be using? Enter your answer using three decimal places (0.114), include zeroes, as needed. Include the correct abbreviation for moles: mol A procedure directs you to weigh 33.607 mmols of dimethyl malonate (M.W. 132.1) into 50mL round-bottom flask. How many grams will you need? Enter your answer using three decimal places (6.807), include zeroes, as needed. Include the correct abbreviation for the appropriate unit
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


