Question: I've provided the entire experiment procedure. Can you please answer the 4 question. Thank you in advance PART A Procedure: In this part of the



I've provided the entire experiment procedure. Can you please answer the 4 question. Thank you in advance
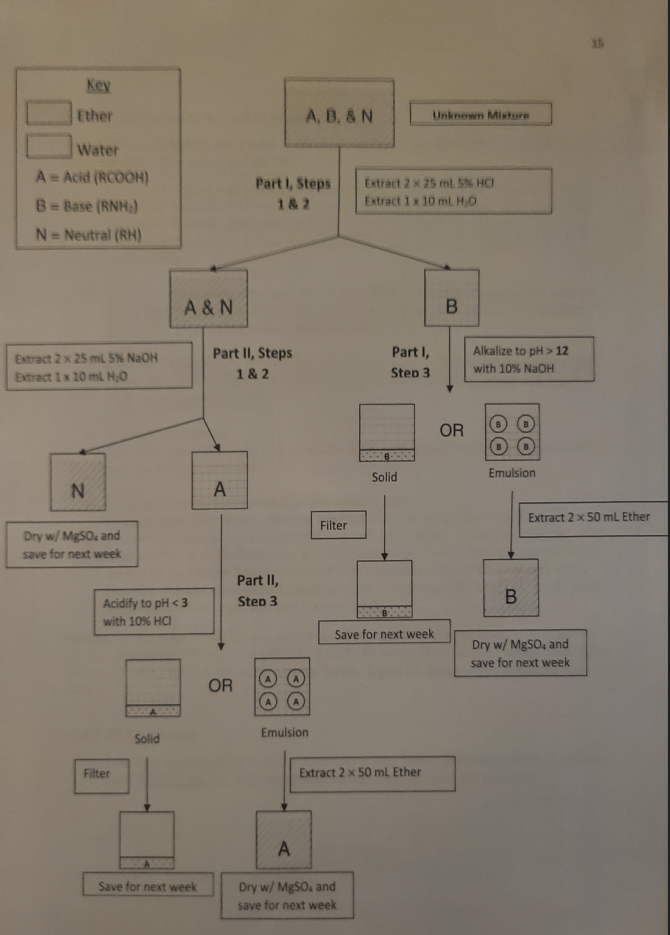
PART A Procedure: In this part of the experiment, a mixture is containing either an acidic or basic compound, and a neutral compound is to be separated into the individual components. The flow chart on the next page will coordinate these steps. Pour the unknown solution into 2250mL separatory funnel. Rinse the Erlettmeyer flask with 5mL of ether and add this rinse to the separatory funnel. PART I Extraction of the base (if present): 1. Extract the organic base (amine) from the organic mixture using two 25mL portions of 5% hydrochloric acid. Draw off the lowel aqueous layer, leaving the upper organic layer, for each extraction and combine these aqueous layers into the same 125mL Erlenmeyer flask. This will be your ORGANIC BASE EXTRACTION. Label appropriately. NOTE: If you observe a large amount of precipitation in your separatory funnel, draw off this precipitation with the aqueous layer. Extract the ether layer with an additional 25mL of water followed by another extraction with 25mL of 5% hydrochloric acid. Combine all aqueous extracts into a 250mL Erlenmeyer and label this ORGANIC BASE EXTRACTION. 2. Extract the ether in the separatory funnel with 10mL of water and combine this with the rest of the ORGANIC BASE EXTRACTION, leaving the ether layer in the separatory Junnel. Set the separatory funnel aside for later. 3. Chill the ORGANIC BASE EXTRACTION flask in an ice bath and make it very basic (raise the pH ) by adding 10% sodium hydroxide until the solution is very alkaline (pH>12). Chill the mixture thoroughly. If a solid precipitates: Seat filter paper with water and collect the solid (organic base) by Suction filtration and save the solid forrext weed If an emulsion occurs (or even if a clear solution persists): In a new separatory funnel, place the emulsion solution and extract with 50m - of ether. Remove the aqueous layer through the stopcack into the beaker or flask and pftr the ether layer out the top of the funnel into a 125mL Erlenmeyer flask. Place the aqueous ayer back in the separatory funnel and extract with a second portion of ether. combine the two ether extracts in the same Erlenmeyer flask. Label thi,ORGANIC BASS dry with magnesium sulfate, cork, and save for next week. 15. NOTE: If you definitely got a solid or emulsion in Part I, you DO NOT need to perform this procedure; skip ahead to Part III. 1. Extract the organic acid (carboxylic) from the ether layer from Part I using two 25mL portions of 5% sodium hydroxide. Draw off the lower aqueous layer, leaving the upper ether layer, for each extraction and combine these aqueous layers into the same 125mL Erlenmeyer flask. This will be your QRGANIC ACID EXTRACTION. Label appropriately. 2. Extract the ether in the separatory funnel with 10mL of water and combine this with the rest of the ORGANIC ACID FXIRACTION, leaving the ether layer in the separatory funnel. Set the separatory funnel aside for later. 3. Chil) the ORGANIC ACID EXTRACTION flask in an ice bath and make it very acidic (lower the pH ) by adding 10% hydrochloric acid until the solution is very acidic ( pH
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


