Question: Just need help with second question. Standard Solutions for FeSCN2+ Beer's Law Plot (calibration) Solution [FeSCN2+1 (x-axis) Absorbance, A (at 447 nm) (y-axis) 0.130 S2
Just need help with second question.
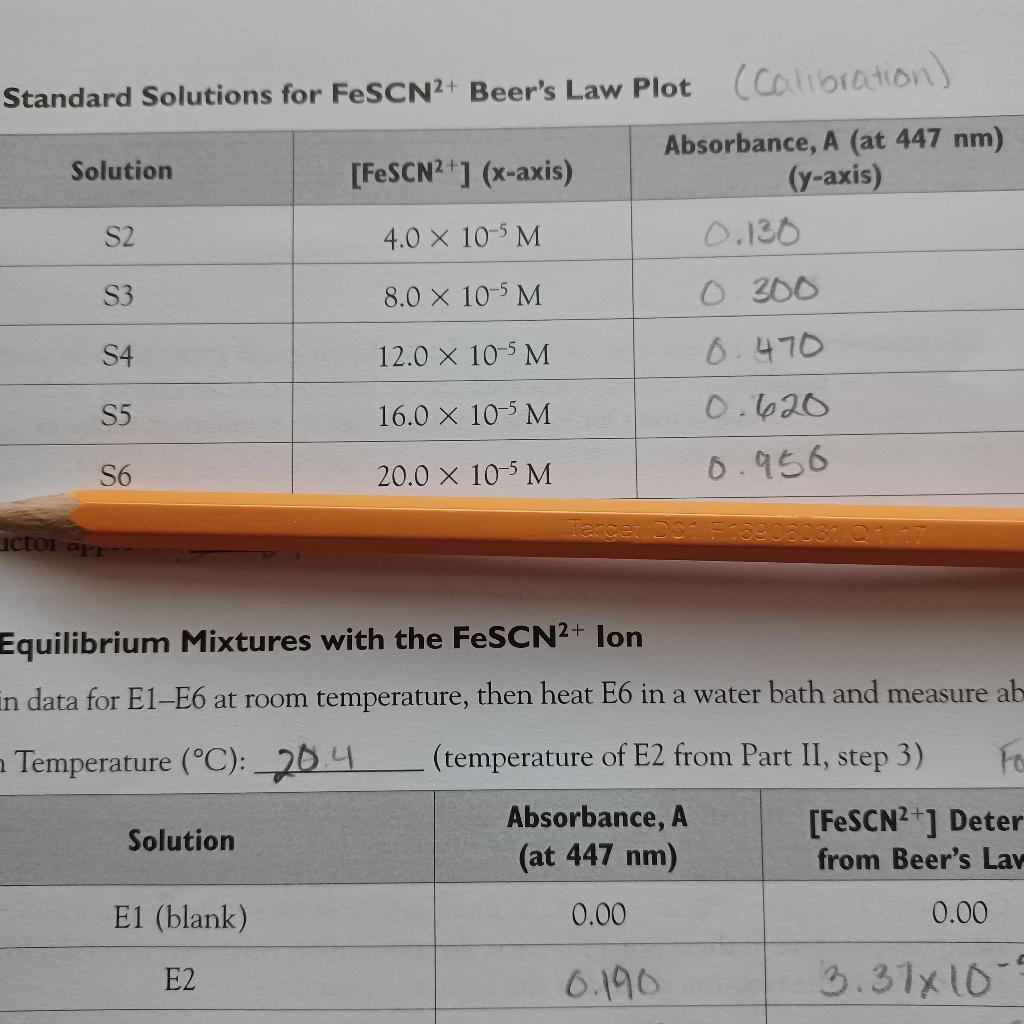

Standard Solutions for FeSCN2+ Beer's Law Plot (calibration) Solution [FeSCN2+1 (x-axis) Absorbance, A (at 447 nm) (y-axis) 0.130 S2 4.0 x 10-5 M S3 8.0 X 10-5 M 0 300 6.470 S4 12.0 x 10-5 M S5 16.0 x 10-5 M 0.620 S6 20.0 x 10-5 M 0.956 ictoi apr Equilibrium Mixtures with the FeSCN2+ lon in data for E1-E6 at room temperature, then heat E6 in a water bath and measure ab Temperature (C): 20.4 (temperature of E2 from Part II, step 3) Fo Absorbance, A [FeSCN2+] Deter Solution (at 447 nm) from Beer's Lav E1 (blank) 0.00 0.00 E2 0.190 3.37x10 Showing your work clearly, answer the following below: 1. Using the data collected to prepare your Beer's law plot, calculate the molar absorptivity constant, e, and its units, for the FeSCN2+ ion at 447 nm. Hint: Select one line of data from your Report Sheet, Part I, and use Equation (3) and b = 1.0 cm. 2. We assumed that all the SCN ion was converted to FeSCN2+ ion in Part I because of the great excess (approximately 1000X) of Fe3+ ion. However, since the equilibrium shown in Equation (27 takes place, a trace amount of SCN ion must also be present. Use your mean K, value to calculate the SCN ion concentration in solution S3. a. b. Based on your answer in Part a, was the assumption made in Part I valid? What percentage SCN ion was converted to the FeSCN2+ ion? Hint: For the assumption to be valid, more the 95% of the SCN ion should have been converted to FeSCN2+ ion
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


