Question: L 3. Answer the questions in reference to the reaction system shown below. A+B 2D r1 = k1CACB2 = = Eai= 30 kJ/mol EA2 2=
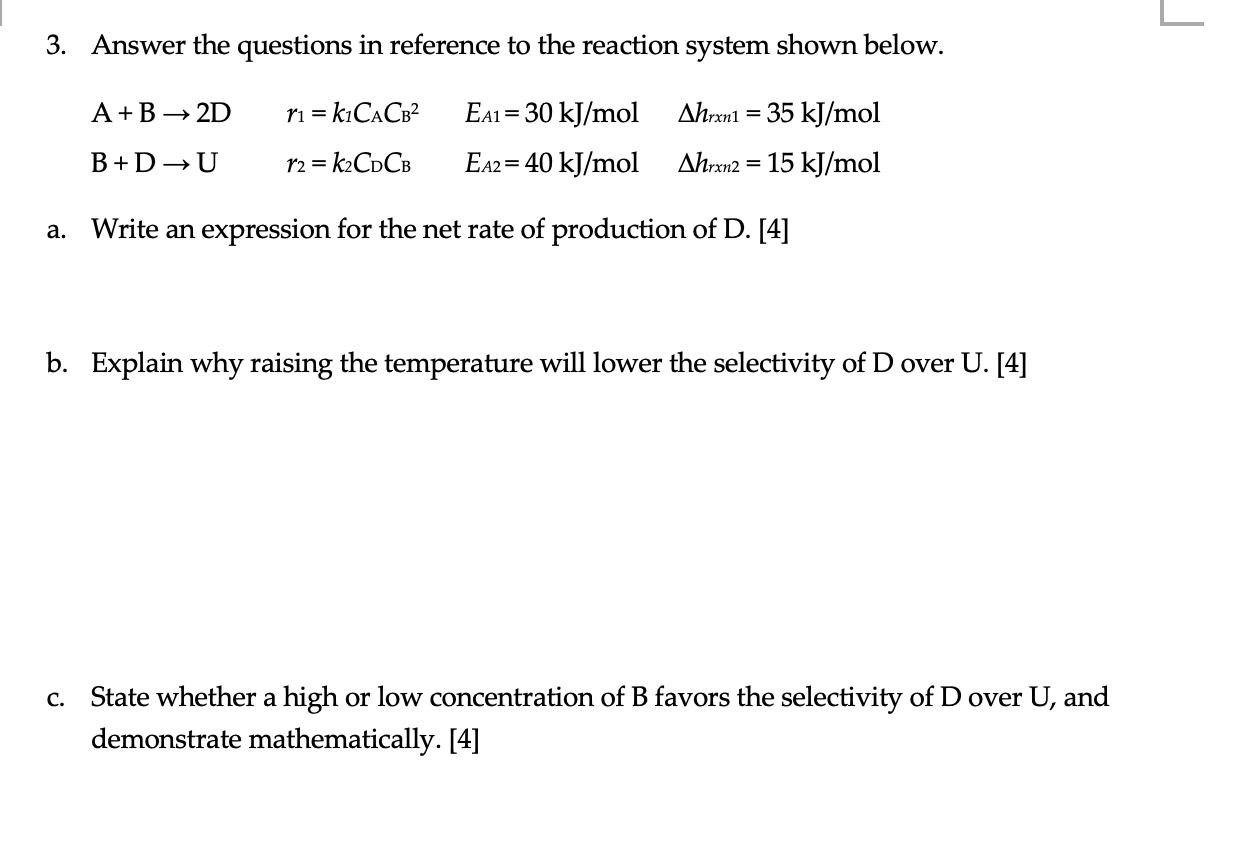
L 3. Answer the questions in reference to the reaction system shown below. A+B 2D r1 = k1CACB2 = = Eai= 30 kJ/mol EA2 2= 40 kJ/mol Ahrxn1 = 35 kJ/mol Ahrxn2 = 15 kJ/mol B+ DU r2 = k2CDCB = a. Write an expression for the net rate of production of D. [4] b. Explain why raising the temperature will lower the selectivity of D over U. [4] C. State whether a high or low concentration of B favors the selectivity of Dover U, and demonstrate mathematically. [4]
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


