Question: & Lab Data Verify your measurement. Raise the eudiometer so that the meniscus is at the level of the water in the beaker. Verify your
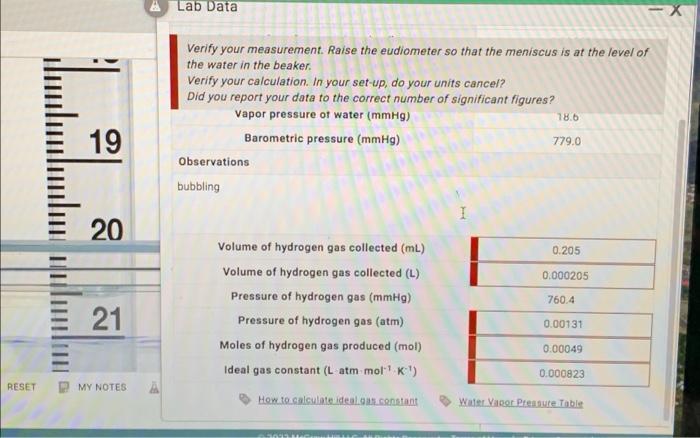
& Lab Data Verify your measurement. Raise the eudiometer so that the meniscus is at the level of the water in the beaker. Verify your calculation. In your set-up, do your units cancel? Did you report your data to the correct number of significant figures? Vapor pressure of water (mmHg) Barometric pressure (mmHg) 779.0 Observations bubbling 78.6 19 20 0.205 0.000205 760.4 21 Volume of hydrogen gas collected (mL) Volume of hydrogen gas collected (L) Pressure of hydrogen gas (mmHg) Pressure of hydrogen gas (atm) Moles of hydrogen gas produced (mol) Ideal gas constant (L atm mol'k') 0.00131 0.00049 0.000823 RESET MY NOTES A How to calculate ideal.c.constant Water Vapor Pressure Table & Lab Data Verify your measurement. Raise the eudiometer so that the meniscus is at the level of the water in the beaker. Verify your calculation. In your set-up, do your units cancel? Did you report your data to the correct number of significant figures? Vapor pressure of water (mmHg) Barometric pressure (mmHg) 779.0 Observations bubbling 78.6 19 20 0.205 0.000205 760.4 21 Volume of hydrogen gas collected (mL) Volume of hydrogen gas collected (L) Pressure of hydrogen gas (mmHg) Pressure of hydrogen gas (atm) Moles of hydrogen gas produced (mol) Ideal gas constant (L atm mol'k') 0.00131 0.00049 0.000823 RESET MY NOTES A How to calculate ideal.c.constant Water Vapor Pressure Table
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


