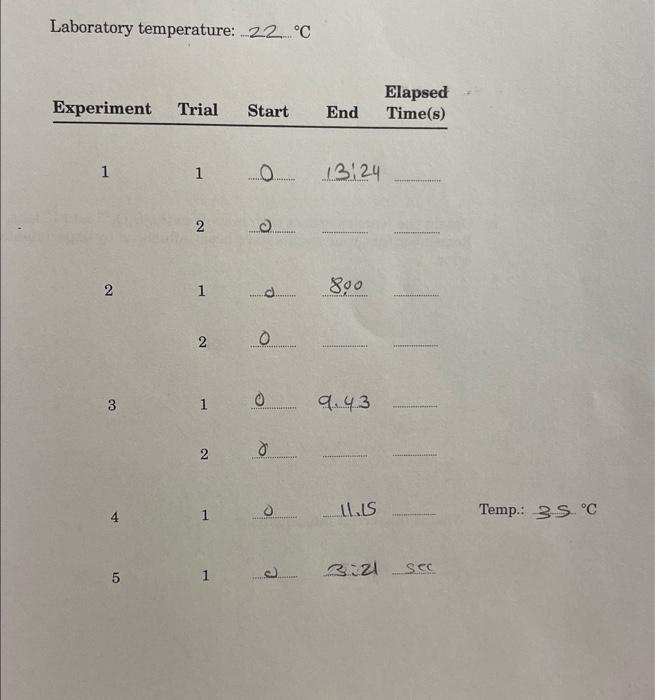
Question: Laboratory temperature: 22C Temp.: 3S 1. a. Calculate the initial rates for Experiments 1,2, and 3 from [S2O82]/t. Use mean elapsed times. Give correct units.
![for Experiments 1,2, and 3 from [S2O82]/t. Use mean elapsed times. Give](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f948ba9fefb_11466f948ba4560b.jpg)


Laboratory temperature: 22C Temp.: 3S 1. a. Calculate the initial rates for Experiments 1,2, and 3 from [S2O82]/t. Use mean elapsed times. Give correct units. b. Obtain the reaction orders with respect to the S2O82 and Iions. Remember that your results have been influenced by experimental error, c. What is the correct rate equation? d. Calculate the initial concentrations of the S2O82 and Iions in Experiments 1,2, and 3 . Remember that dilution occurred when the solutions were prepared. e. Calculate the rate constant in Experiments 1, 2, and 3, and obtain the mean. Give the correct units. 2. a. Calculate the rate constant at the higher temperature used in Experiment 4. b. Calculate the activation energy for the reaction. 3. What do your results demonstrate about the effect of a catalyst on the rate of a reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


