Question: Liquid extraction is an operation used to separate the components of a liquid mixture of two or more species. In the simplest case, the mixture

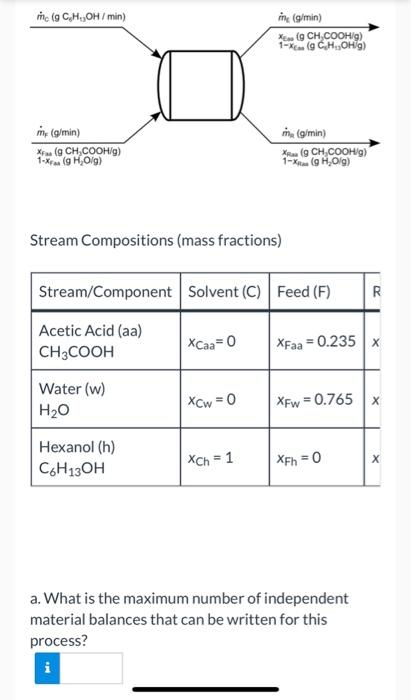
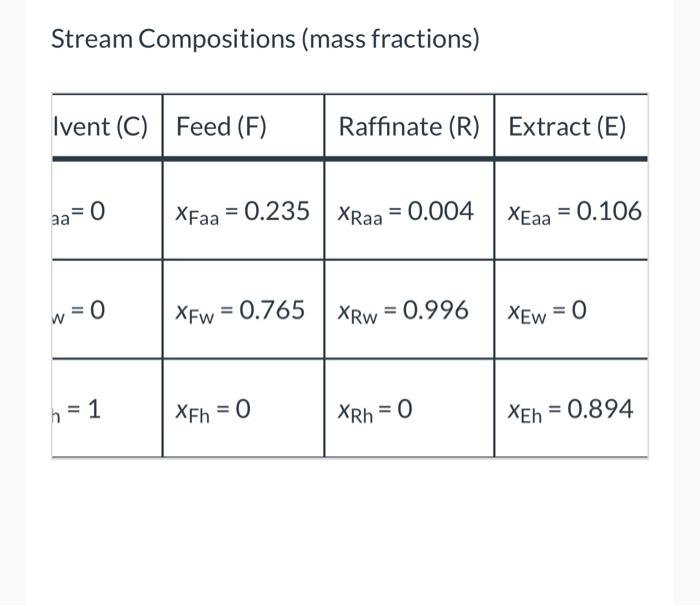
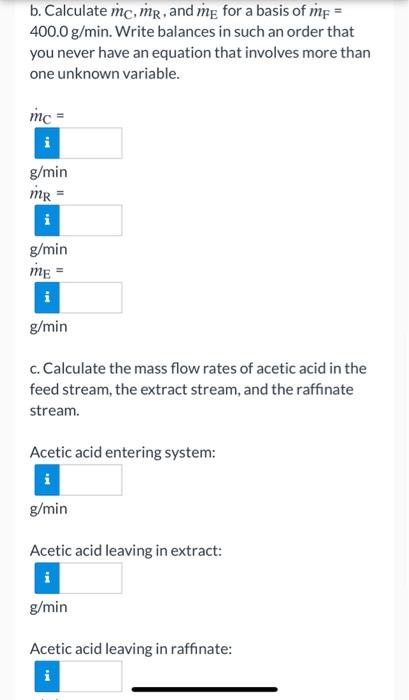
Liquid extraction is an operation used to separate the components of a liquid mixture of two or more species. In the simplest case, the mixture contains two components: a solute (A) and a liquid solvent (B). The mixture is contacted in an agitated vessle with a second liquid solvent (C) that has two key properties: A dissolves in it, and B is immiscible or nearly immiscible with it. (For example, B may be water, C a hydrocarbon oil, and A a species that has significant solubility in both water and oil.) Some of the A transfers from B to C, and then the B-rich phase (the raffinate) and the C- rich phase (the extract) separate from each other in a settling tank. If the raffinate is then contacted with fresh C in another stage, more A will be transferred from it. This process can be repeated until essentially all of the A has been extracted from the B. Shown below is a flowchart of a process in which acetic acid (aa) is extracted from a mixture of acetic acid and water (w) into 1-hexanol (h), a liquid immiscible with water. me (g C.H.,OH/min) mine (g/min) X (CH.COOHg) 1- ( C.HOH) (g/min) ru (CH,COOH/g) 1-X (9H 0 g) mi (g/min) (CH.COOHg) 1-X (HO) Stream Compositions (mass fractions) Stream/Component Solvent (C) Feed (F) DY Acetic Acid (aa) CH3COOH XCaa=0 XFaa=0.235 Water (w) H20 xCw=0 XFw = 0.765 Hexanol (h) CH3OH Xch = 1 XFh = 0 a. What is the maximum number of independent material balances that can be written for this process? Stream Compositions (mass fractions) Ivent (C) Feed (F) Raffinate (R) Extract (E) aa=0 XFaa = 0.235 XRaa = 0.004 XEaa = 0.106 w=0 XFw = 0.765 XRw = 0.996 XEw = 0 h = 1 XEh =0 XRh = 0 XEh = 0.894 b. Calculate mc, mr, and me for a basis of mp = 400.0 g/min. Write balances in such an order that you never have an equation that involves more than one unknown variable. mc = i g/min mr = g/min me = g/min c. Calculate the mass flow rates of acetic acid in the feed stream, the extract stream, and the raffinate stream. Acetic acid entering system: g/min Acetic acid leaving in extract: g/min Acetic acid leaving in raffinate: i c. Calculate the mass flow rates of acetic acid in the feed stream, the extract stream, and the raffinate stream. Acetic acid entering system: g/min Acetic acid leaving in extract: g/min Acetic acid leaving in raffinate: g/min e Textbook and Media Save for Later Attempts: 0 of 3 used Submit
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


