Question: Matlab problem: Program: assign03.m Calculate the pressure exerted by 2 mol of water vapor (steam) in a 1 L container at 2000 F using the
Matlab problem: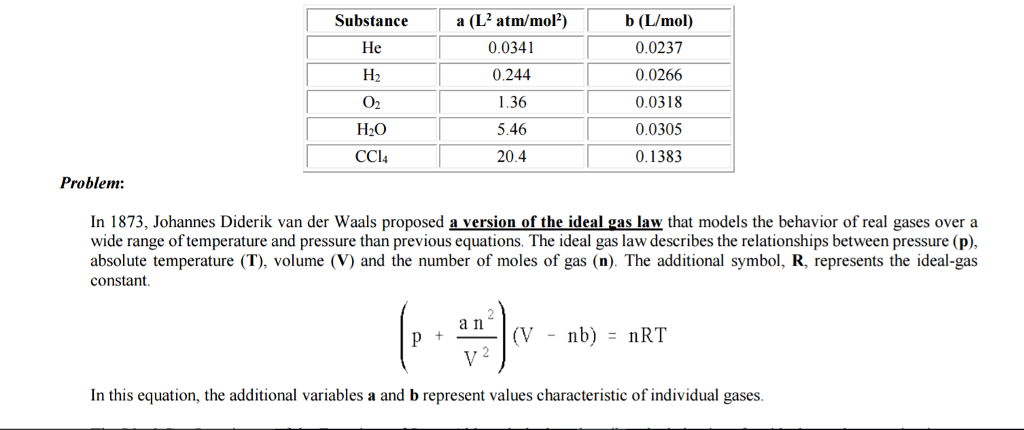 Program: assign03.m Calculate the pressure exerted by 2 mol of water vapor (steam) in a 1 L container at 2000 F using the Van der Waals equation and the Ideal Gas Law equation. What is the difference between the non-ideal and ideal gases?
Program: assign03.m Calculate the pressure exerted by 2 mol of water vapor (steam) in a 1 L container at 2000 F using the Van der Waals equation and the Ideal Gas Law equation. What is the difference between the non-ideal and ideal gases?
We know that : temperature=2000 F moles, n=2 mol volume, V=1 L ideal gas constant, R=0.08314472 L atm/K mol.
Here are the current result(output), but I don't know how the process in Matlab.I tried many timmes, it shows error all the time...
Thanks for you helpI appreciate it.
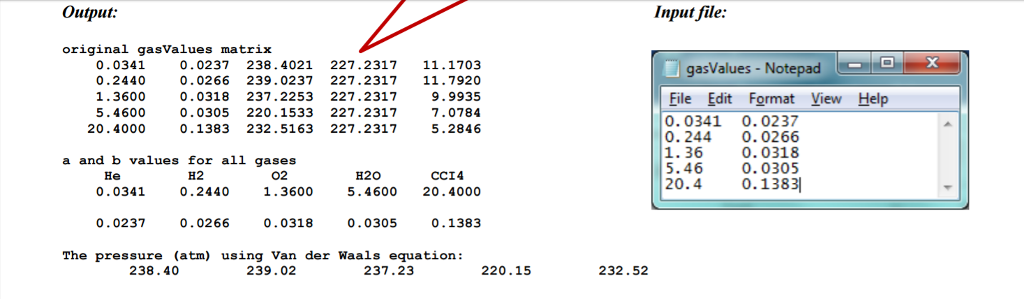
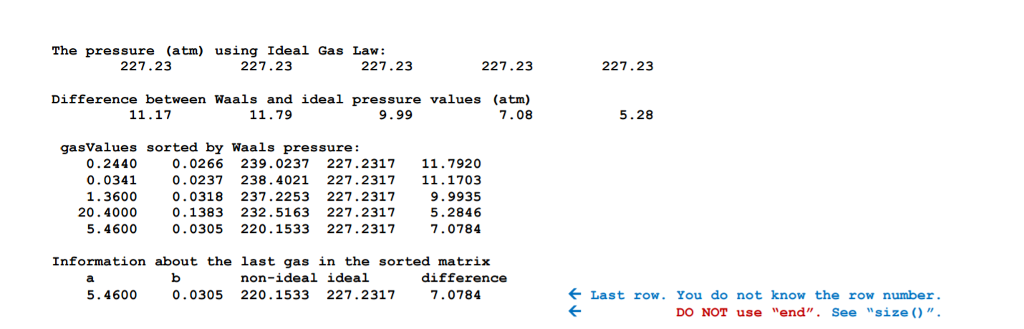
Substance a (L2 atm/molz) b (L/mol) 0.0341 0.0237 He H2 0.0266 0.244 0.0318 1.36 0.0305 H2O 5.46 0.1383 CC14 20.4 Problem: In 1873, Johannes Diderik van der Waals proposed a version of the ideal gas law that models the behavior of real gases over a wide range of temperature and pressure than previous equations. The ideal gas law describes the relationships between pressure (p), constant a n nb) RT In this equation, the additional variables a and b represent values characteristic of individual gases
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


