Question: mn = 0.59 V, E, = -3.04 V I) Calculate AG (kJ/mol) for the following electrochemical cell II) If the cell emf of a
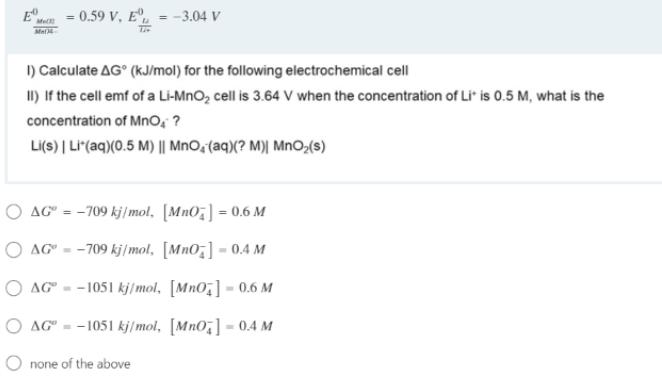
mn = 0.59 V, E, = -3.04 V I) Calculate AG (kJ/mol) for the following electrochemical cell II) If the cell emf of a Li-MnO, cell is 3.64 V when the concentration of Li" is 0.5 M, what is the concentration of Mno, ? Li(S) | LI(aq)(0.5 M) || MnO, (aq)(? M)| MnO,(s) O AG" = -709 kj/mol, [MnO;] = 0.6 M O AG" - -709 kj/mol, [Mno,] - 0.4 M O AG" -- 1051 kj/mol, [Mno,] - 0.6 M O AG" - -1051 kj/mol, [Mn0,] 0.4 M O none of the above
Step by Step Solution
3.46 Rating (156 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
60d452b082dfa_227638.pdf
180 KBs PDF File
60d452b082dfa_227638.docx
120 KBs Word File


