Question: Must solve using MATLAB (if you can copy and paste code here that would be best) The heat capacity at constant pressure (C_p) is the
Must solve using MATLAB (if you can copy and paste code here that would be best)
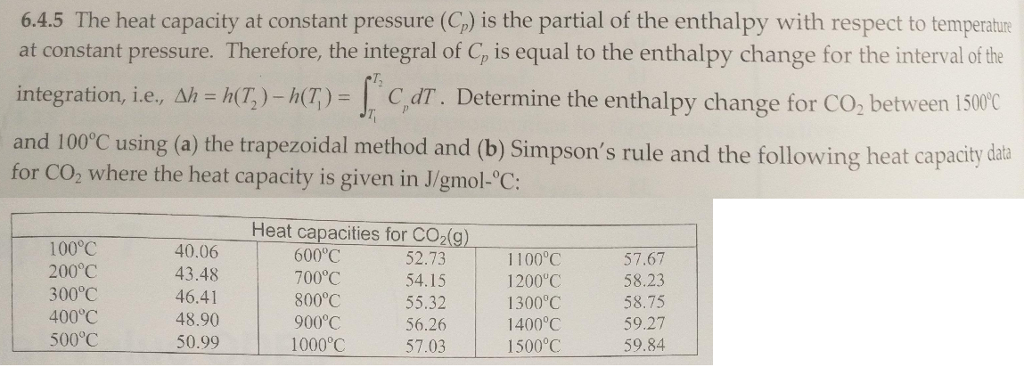
The heat capacity at constant pressure (C_p) is the partial of the enthalpy with respect to temperature at constant pressure Therefore, the integral of C_p is equal to the enthalpy change for the interval of the integration, i.e., Delta h = h(T_2) - h_1(T_1) = integral^T_2_T_1 C_p dT. Determine the enthalpy change for CO_2 between 1500 degree C and 100 degree C using (a) the trapezoidal method and (b) Simpson's rule and the following heat capacity data for CO_2 where the heat capacity is given in J/gmol- degree C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


