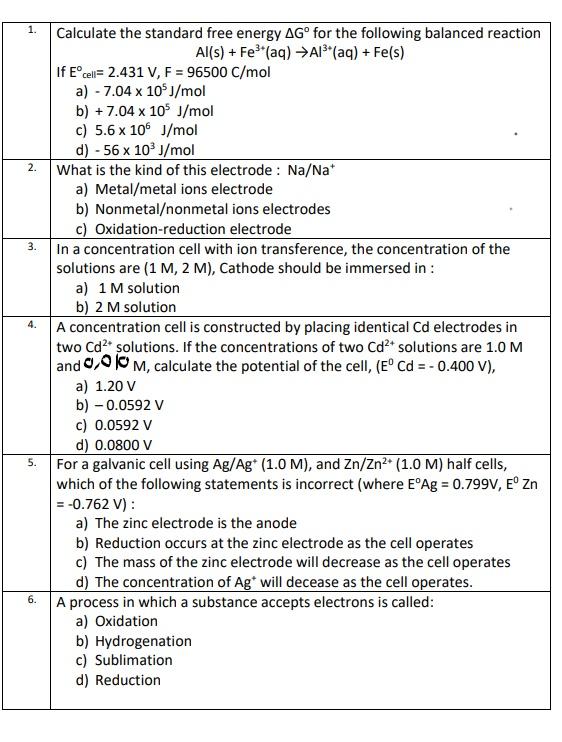
Question: need all questions multiple choice question 1. 2. 3. 4. Calculate the standard free energy AG for the following balanced reaction Al(s) + Fes(aq) A13+
 need all questions multiple choice question
need all questions multiple choice question
1. 2. 3. 4. Calculate the standard free energy AG for the following balanced reaction Al(s) + Fes"(aq) A13+ (aq) + Fe(s) If Ecell = 2.431 V, F = 96500 C/mol a) - 7.04 x 10$/mol b) +7.04 x 10 J/mol c) 5.6 x 106 J/mol d) - 56 x 103 J/mol What is the kind of this electrode : Na/Nat a) Metal/metal ions electrode b) Nonmetalonmetal ions electrodes c) Oxidation-reduction electrode In a concentration cell with ion transference, the concentration of the solutions are (1 M, 2 M), Cathode should be immersed in : a) 1 M solution b) 2 M solution A concentration cell is constructed by placing identical Cd electrodes in two Cd2+ solutions. If the concentrations of two Cd2+ solutions are 1.0 M and 0,010 M, calculate the potential of the cell, (ECd = - 0.400 V), a) 1.20 V b) -0.0592 v c) 0.0592 v d) 0.0800 v For a galvanic cell using Ag/Ag* (1.0 M), and Zn/Zn2+ (1.0 M) half cells, which of the following statements is incorrect (where EAg = 0.799V, E Zn = -0.762 V): a) The zinc electrode is the anode b) Reduction occurs at the zinc electrode as the cell operates c) The mass of the zinc electrode will decrease as the cell operates d) The concentration of Ag* will decease as the cell operates. A process in which a substance accepts electrons is called: a) Oxidation b) Hydrogenation c) Sublimation d) Reduction 5. 6
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


