Question: Need help with the empty spaces Calculations for the anhydrous sample Mass of sample (g) 0.5 First heating Mass of crucible, lid, and anhydrous sample
Need help with the empty spaces 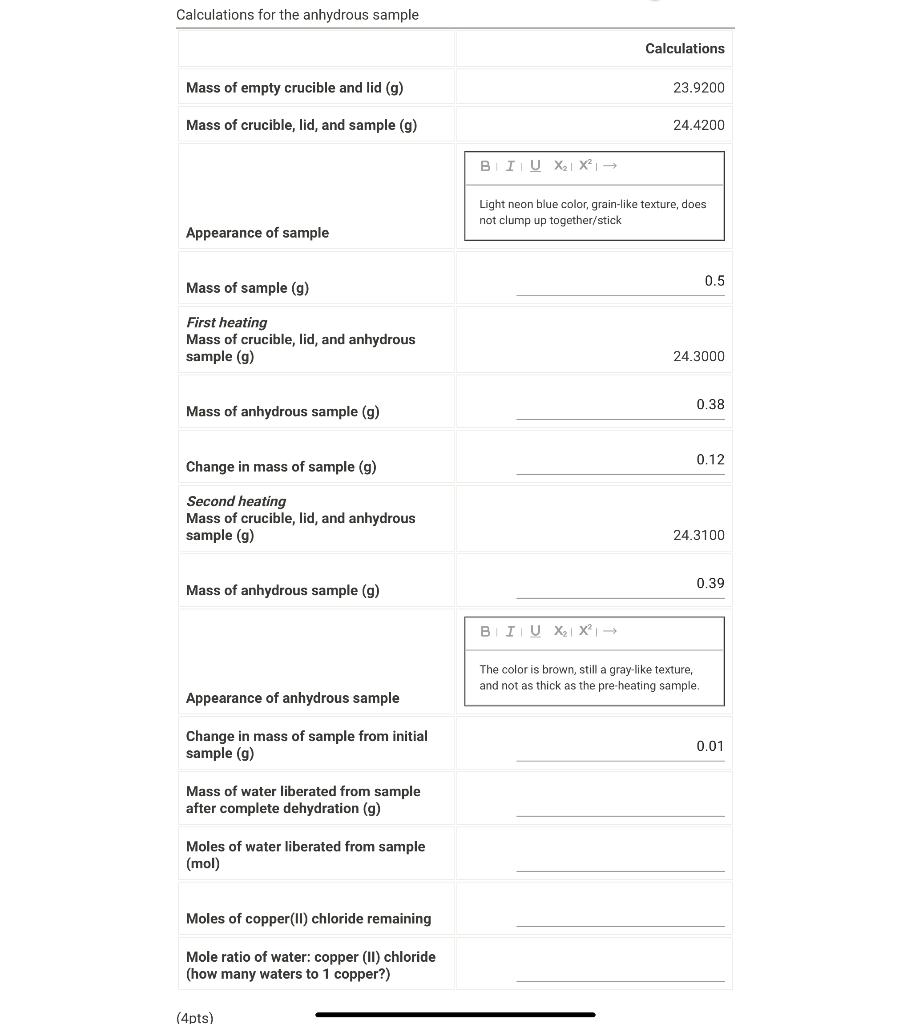
Calculations for the anhydrous sample Mass of sample (g) 0.5 First heating Mass of crucible, lid, and anhydrous sample (g) 24.3000 Mass of anhydrous sample (g) 0.38 Change in mass of sample (g) 0.12 Second heating Mass of crucible, lid, and anhydrous sample (g) 24.3100 Mass of anhydrous sample (g) Change in mass of sample from initial sample (g) Mass of water liberated from sample after complete dehydration (g) Moles of water liberated from sample (mol) Moles of copper(II) chloride remaining Mole ratio of water: copper (II) chloride (how many waters to 1 copper?)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


