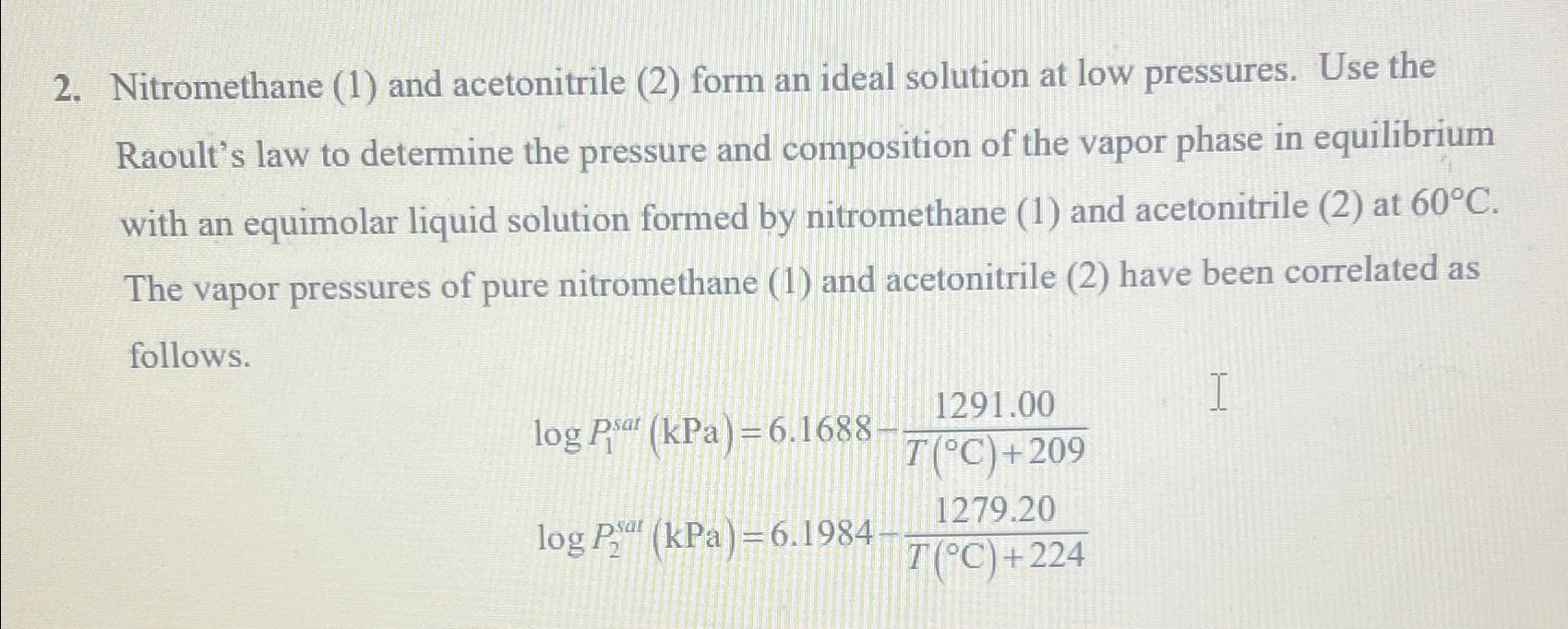
Question: Nitromethane ( 1 ) and acetonitrile ( 2 ) form an ideal solution at low pressures. Use the Raoult's law to determine the pressure and
Nitromethane and acetonitrile form an ideal solution at low pressures. Use the Raoult's law to determine the pressure and composition of the vapor phase in equilibrium with an equimolar liquid solution formed by nitromethane and acetonitrile at The vapor pressures of pure nitromethane and acetonitrile have been correlated as follows.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


