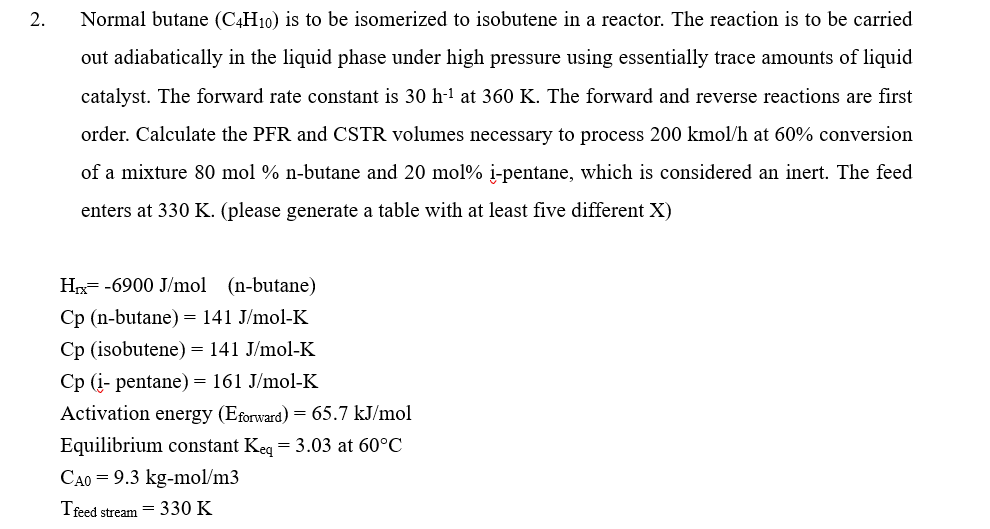
Question: Normal butane ( C 4 H 1 0 ) is to be isomerized to isobutene in a reactor. The reaction is to be carried out
Normal butane is to be isomerized to isobutene in a reactor. The reaction is to be carried
out adiabatically in the liquid phase under high pressure using essentially trace amounts of liquid
catalyst. The forward rate constant is at The forward and reverse reactions are first
order. Calculate the PFR and CSTR volumes necessary to process kmo at conversion
of a mixture mol nbutane and mol ipentane, which is considered an inert. The feed
enters at please generate a table with at least five different X
butane
butane
pentane
Activation energy
Equilibrium constant
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


