Question: ) On the graph below, draw a reaction energy profile of a reaction with the following: Reaction Progress Now add a dotted line that represents
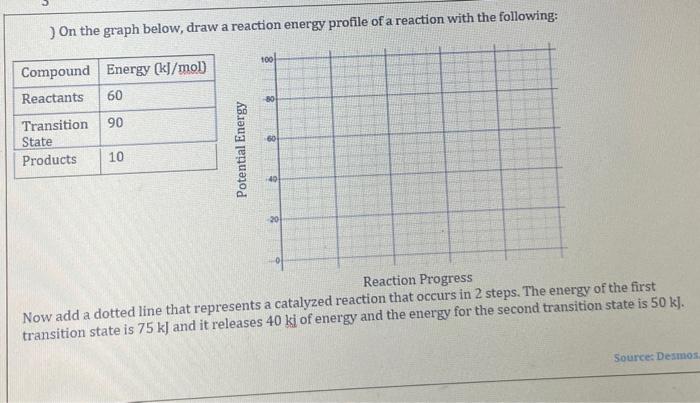
) On the graph below, draw a reaction energy profile of a reaction with the following: Reaction Progress Now add a dotted line that represents a catalyzed reaction that occurs in 2 steps. The energy of the first transition state is 75k ] and it releases 40ki of energy and the energy for the second transition state is 50kJ
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


