Question: One Component Diffusing in Another Stagnant Component In Figure shown below, an open beaker, 6cm high, is filled with liquid benzene (A) at 25C to
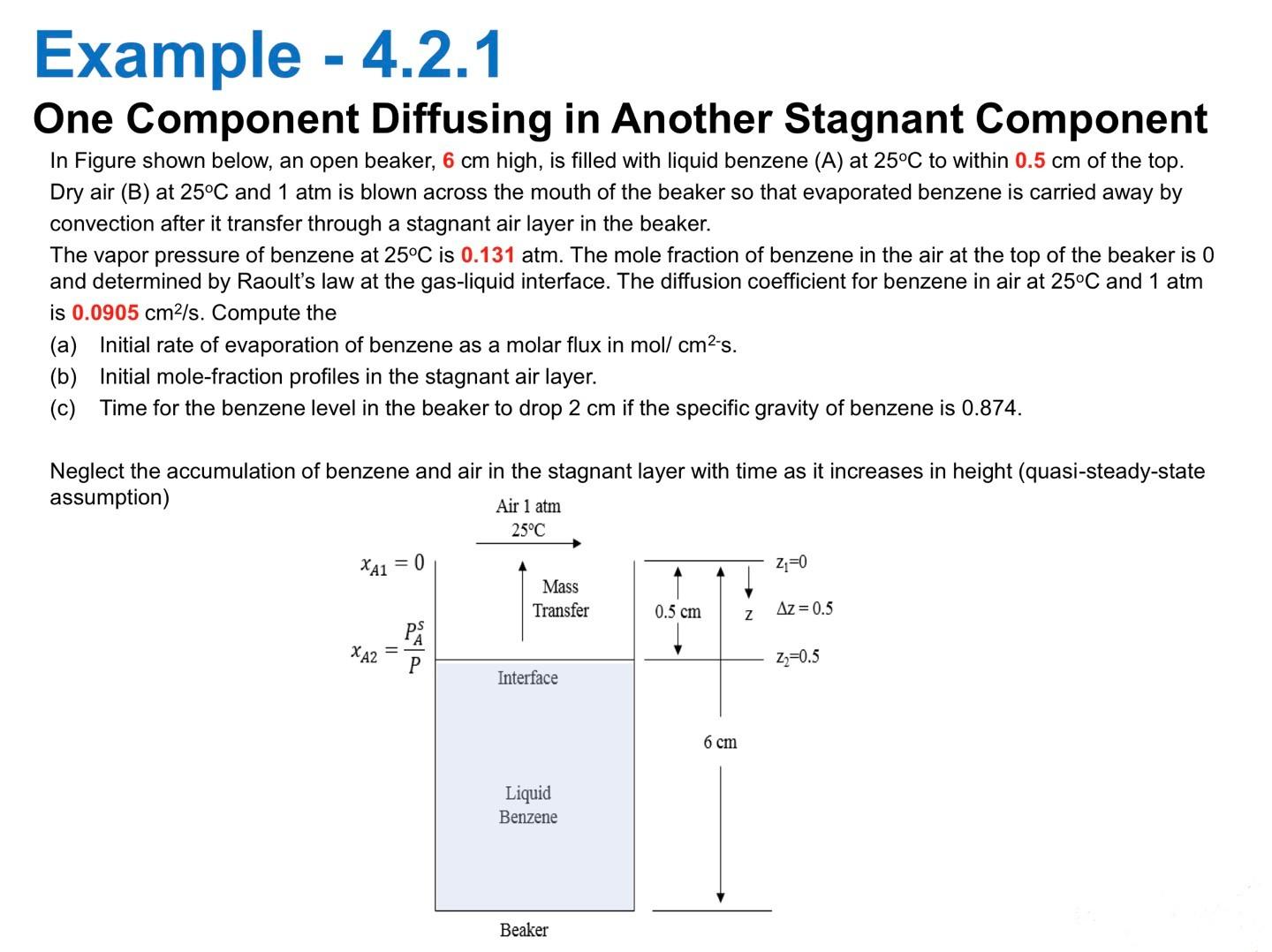

One Component Diffusing in Another Stagnant Component In Figure shown below, an open beaker, 6cm high, is filled with liquid benzene (A) at 25C to within 0.5cm of the top. Dry air (B) at 25C and 1atm is blown across the mouth of the beaker so that evaporated benzene is carried away by convection after it transfer through a stagnant air layer in the beaker. The vapor pressure of benzene at 25C is 0.131atm. The mole fraction of benzene in the air at the top of the beaker is 0 and determined by Raoult's law at the gas-liquid interface. The diffusion coefficient for benzene in air at 25C and 1 atm is 0.0905cm2/s. Compute the (a) Initial rate of evaporation of benzene as a molar flux in mol/cm2s. (b) Initial mole-fraction profiles in the stagnant air layer. (c) Time for the benzene level in the beaker to drop 2cm if the specific gravity of benzene is 0.874 . Neglect the accumulation of benzene and air in the stagnant layer with time as it increases in height (quasi-steady-state 3. Solve Example 4.2.1 (a) \& (c) in the on-line lecture: Maintain all conditions same as Example 4.2.1 but change the material from Benzene to Methanol (it means you must evaluate Methanol properties needed for solving Example). The diffusion coefficient for Methanol in air at 25C and 1 atm is assumed as 0.4cm2/s. The specific gravity of Methanol is 0.8 . Vapor pressure of Methanol can be calculated using Antoine E
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


