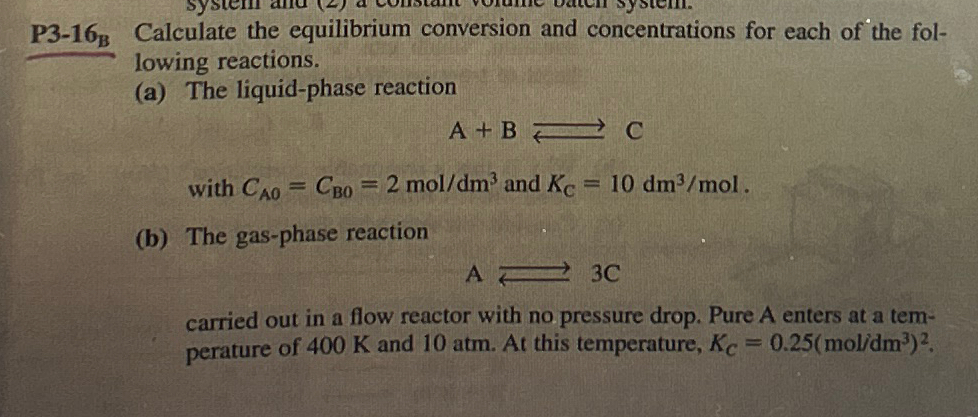
Question: P 3 - 1 6 ? B Calculate the equilibrium conversion and concentrations for each of the following reactions. ( a ) The liquid -
P Calculate the equilibrium conversion and concentrations for each of the following reactions.
a The liquidphase reaction
with and
b The gasphase reaction
carried out in a flow reactor with no pressure drop. Pure A enters at a temperature of and atm. At this temperature,
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


