Question: Problem 1 (25 points) Calculate the equilibrium conversion and concentrations for the reactions below that follow elementary rate law: (a) The liquid-phase reaction: A+B=C with
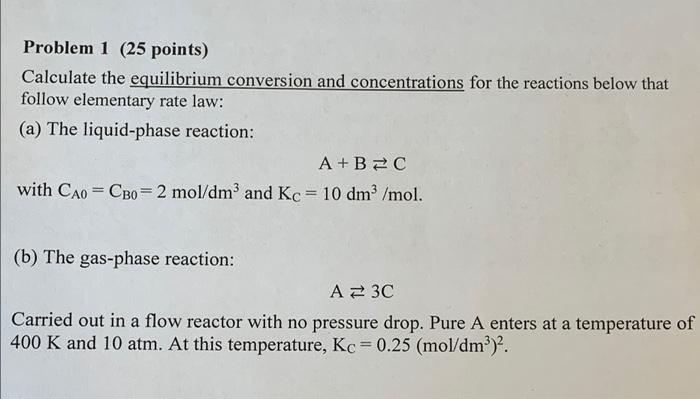
Problem 1 (25 points) Calculate the equilibrium conversion and concentrations for the reactions below that follow elementary rate law: (a) The liquid-phase reaction: A+B=C with Cao = CBo = 2 mol/dm3 and Kc = 10 dm3/mol. (b) The gas-phase reaction: AZ 3C Carried out in a flow reactor with no pressure drop. Pure A enters at a temperature of 400 K and 10 atm. At this temperature, Kc = 0.25 (mol/dm)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


