Question: Part A, B, and C please Compute the specific gas constant for a gas which is composed by 70% oxygen and 30% nitrogen (in mass),
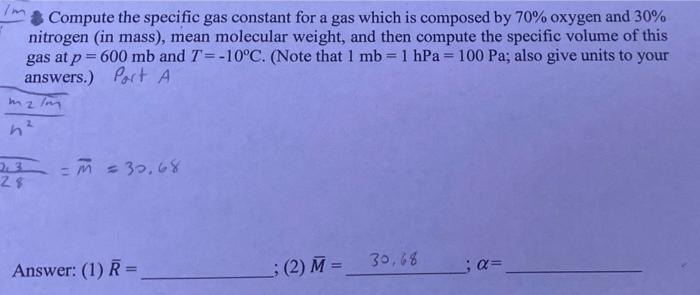
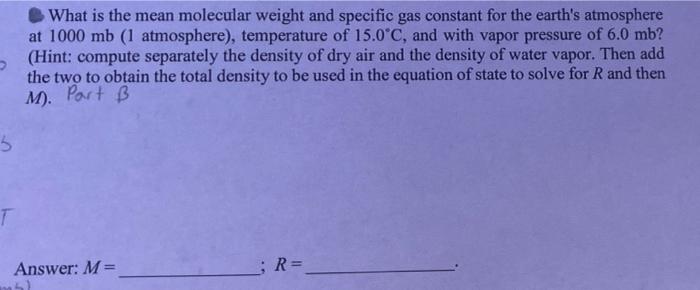
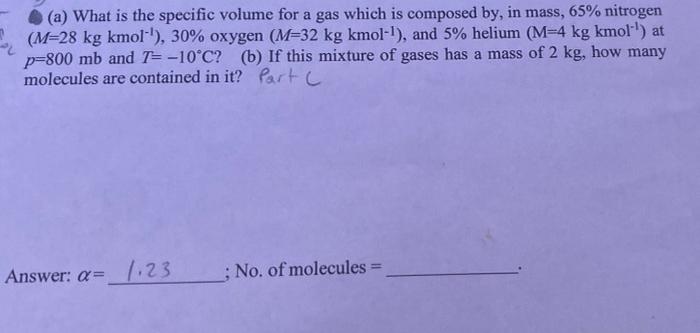



Compute the specific gas constant for a gas which is composed by 70% oxygen and 30% nitrogen (in mass), mean molecular weight, and then compute the specific volume of this gas at p = 600 mb and T=-10C. (Note that I mb=1 hPa = 100 Pa; also give units to your answers.) Port A malm z n 23 28 M = 39,68 Answer: (1) R = (2) M = 30,68 ;a= What is the mean molecular weight and specific gas constant for the earth's atmosphere at 1000 mb (1 atmosphere), temperature of 15.0*C, and with vapor pressure of 6.0 mb? (Hint: compute separately the density of dry air and the density of water vapor. Then add the two to obtain the total density to be used in the equation of state to solve for R and then M). Part B 3 Answer: M= ; R= (a) What is the specific volume for a gas which is composed by, in mass, 65% nitrogen (M=28 kg kmol"'), 30% oxygen (M=32 kg kmol-!), and 5% helium (M-4 kg kmol!) at 2 p=800 mb and T= -10C? (b) If this mixture of gases has a mass of 2 kg, how many molecules are contained in it? Partic Answer: a= 7:23 ; No. of molecules = # 101 62 (M = 1.TXT My 1 M 7%C2 *** X for this ertne gas Tread, 7-17 | . or 7r A: Moutair R* BES #2 M=? R=V ale Earth Atmos: (1000mb), 150 Py = komb # (1 mb=100 Pa) 0 Stone (1000mb) P=ART T (36th) ist 156 994m , wy 6.0 mb Pu P-BRT PERT M Hint: So A. Me M 2)- 2 - 07 A3 72 + 20 univasd gas const, R=R mostar BE #3 (mixture of gas) & ( (65% Ne #2 (M = ?) R = V ooo mb. 15C 30% O2 5% He Earth Atmos Py = komb R= alle (Imba ( P = Poomb P + Py m= 1 home (sm) PAART 7 760 T = -10C 6.0 mb 994 P = LRT PLERT = (Sa+hr) Hint: S. lv PORT @ To en
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


