Question: Part A. Calculate the enthalpy change for the process in which 33.8 g of water is converted from liquid at 0.3 degrees C to vapor
Part A.
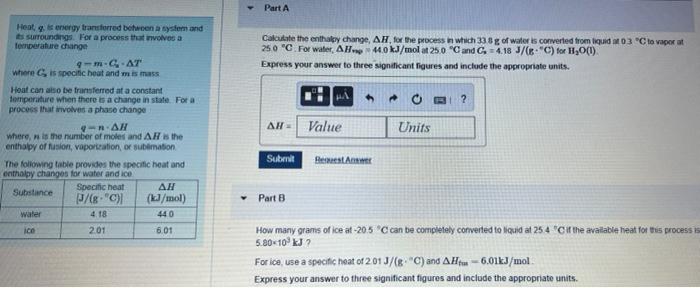
Part A Calculate the enthay change, AH, for the process in which 33 g of water is converted from liquidM03 "Cto vapor at 25.0 "C. For water, AH 440 kJ/molt 250 "Cand C.-4.18 J/("C) for H2O(1) Express your answer to three sindicant figures and include the appropriate units. BI? Heat energy transferred between a system and its surroundings. For a process that involves a temperature change 9 ---AT Where is specific heat and mismas Hoat conso be transferred at a constant forriperture when there is a change in state Fora process that involves a phase change ORAN Wheren is the number of more and AR Is the enthalpy of fusion, voportation, or sublimation The following table provides the specific heat and enthalpy changes for water and ico Substance Specific heat J/8 "C) (1.3/mol) water 4.18 440 ico 2.01 6.01 AW Value Units Submit BAM Part 8 How many grams of ice at -20.5 C can be completely converted to liquid at 254 "Cif the available heat for this process is 5.80-10 kJ For ice, use a specific heat of 201 J/(C) and AH-6.01kJ/mol Express your answer to three significant figures and include the appropriate units
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


