Question: part b and c please A sodium chloride solution containing 120g of water that has a melting point of 2.0C. Assuming the vant Hott factors
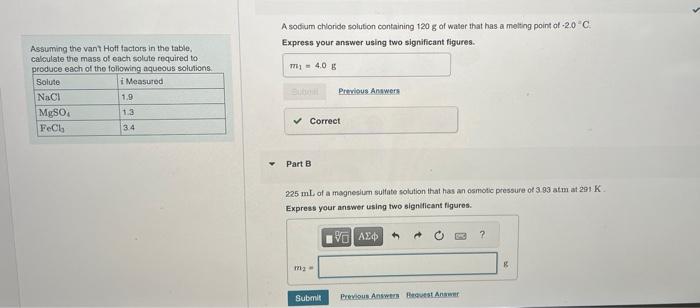
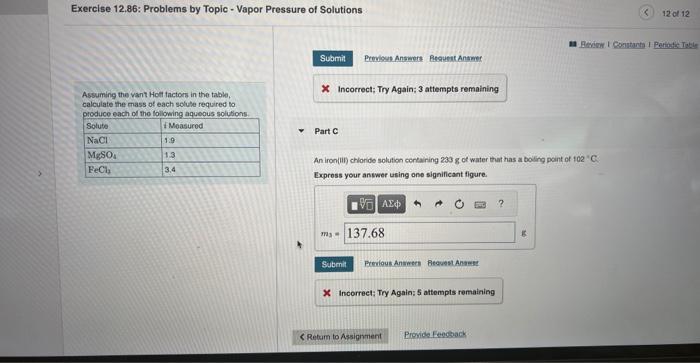
A sodium chloride solution containing 120g of water that has a melting point of 2.0C. Assuming the vant Hott factors in the table, Express your answer using two significant figures. calculate the mass of each solute required to. nrndica aach of the followina aaueous solutions. Part B 225mL of a magneslum sulfate solution that has an osmotic pressure of 3.93 atm at 291K. Express your answer using two signeficant figures. Exercise 12,86: Problems by Topic - Vapor Pressure of Solutions Assuming the vant Hot factors in the table, 14. Incorrect; Try Again; 3 attempte remaining calculate the mass of each solule required to atoduce each of the followina agueaus solutions Part C An iron(ii) chloride solution cortaining 233 g. of water that has a boiling peint of 102 " C. Expross your answer using one significant figure. X Incorreet: Try Agaia: 5 attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


