Question: Phosphine ( P H 3 ) can theoretically be made by the reaction of hydrogen gas and red phosphorus by the reaction below. What temperature
Phosphine can theoretically be made by the reaction of hydrogen gas and red phosphorus by the reaction below. What temperature conditions are required above or below what temperature for the formation of to be favored at standard pressure? note that is per mole
red
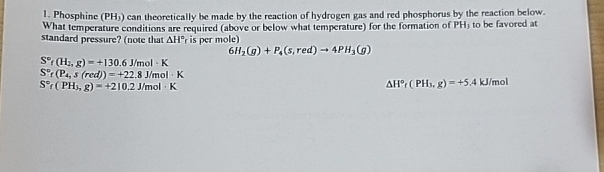
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


